- Home
- Resarch
- Research Groups
- Prof. Arjan W. Kleij
Prof. Arjan W. Kleij
Catalytic, sustainable and selective organic synthesis and biopolymer development
Research Overview
The research of the group is divided over three major objectives. Each of these objectives is briefly outlined below and accompanied by a typical example of a contribution that shows how we wish to contribute to the advancement of the area using (homogeneous) catalysis as a key enabling technology.
CO2 valorization catalysis
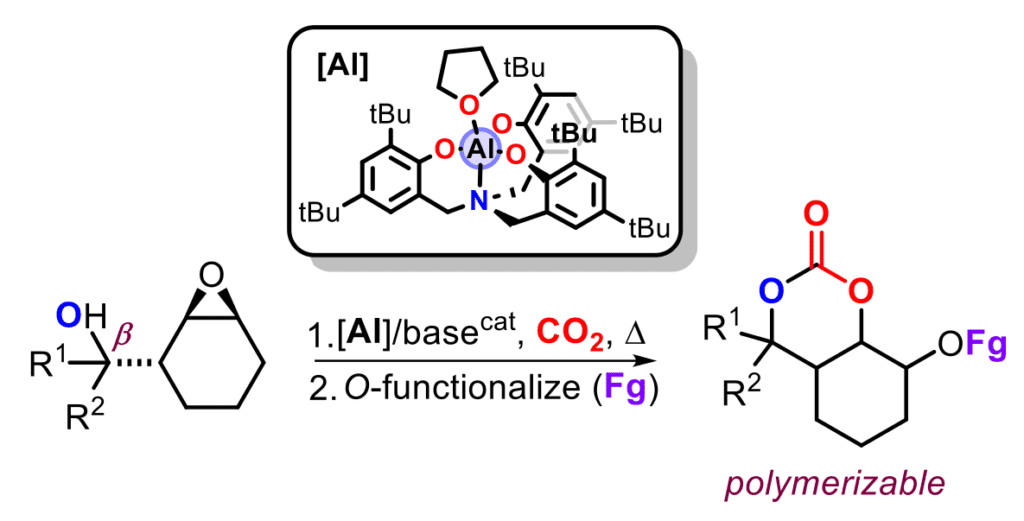
The reuse of carbon renewables such as carbon dioxide is important in the design of low-carbon or even carbon-neutral technologies that are part of a more sustainable future. We have been active for over a decade in the catalytic nonreductive conversion of CO2 into various products among which are diverse heterocycles. Apart from conventional and somewhat limited coupling of oxiranes and CO2 providing cyclic carbonates, we have further extended the synthetic potential of such scaffolds by designing a new conceptual approach for the activation and transformation of CO2 making use of bifunctional substrates (such as epoxy-alcohols and -amines). Within this manifold, the initial activation of CO2 occurs through a hemicarbonate or –carbamate species that subsequently enables an intramolecular ringopening of the epoxide. This novel pathway has allowed to design new molecular complexity from relatively simple building blocks and expanded the repertoire of functionalized CO2-based heterocyclic synthons. A representative example is shown in the Figure, with a bicyclic six-membered cyclic carbonate produced via the abovedetailed route while providing new polymerizable monomers leading to novel polycarbonate macromolecules.
Biopolymer development
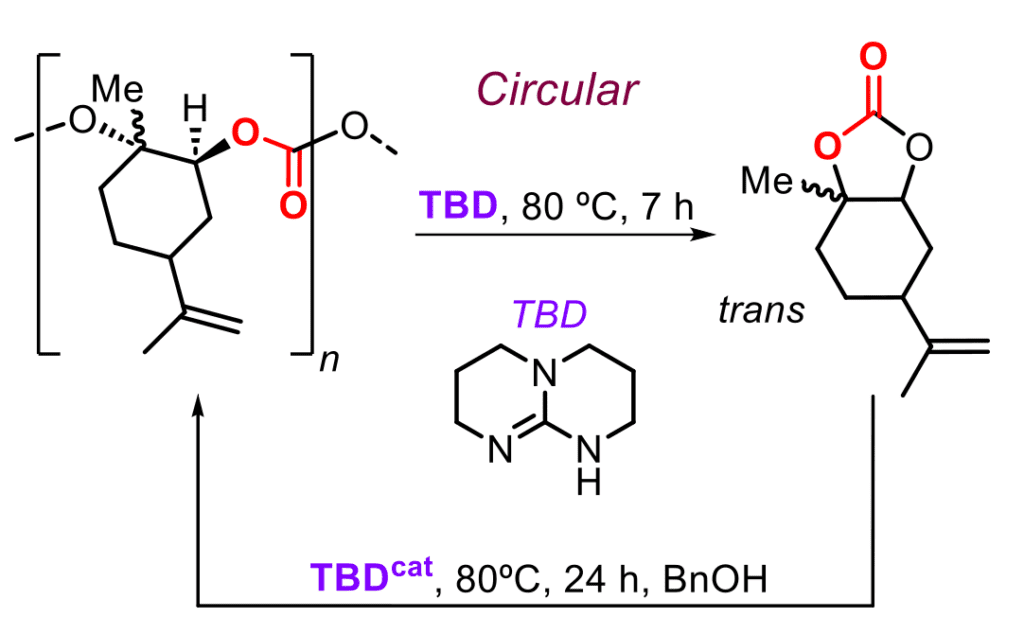
In this context, we have designed metal catalysts derived from aminotriphenolate ligands (M = Al, Fe, Co, Mn, Cr) and used these in combination with suitable initiators to promote the ring-opening polymerization (ROCOP) of sterically challenging terpene and fatty acid based epoxides with either carbon dioxide (to give polycarbonates) and cyclic anhydrides (to produce polyesters) with a high level of functionality and structural diversity. One very much advanced biopolymer that we developed over the years is poly(limonene carbonate) obtained from Dlimonene, which has been investigated in terms of scalability, post-synthetic manipulation, as a replacement in resin-type applications and applied as additive in adhesive materials. Furthermore, in a recent embodiment of our work we showed that under appropriate organocatalytic conditions, this polymer can be selectively degraded into trans-limonene carbonate (a cyclic product; see Figure), whose successful use in ring-opening polymerization regenerated the polycarbonate. A new type of degradation mechanism was discovered that enforces the view that catalysis is crucial in the development of new types of recyclable plastics, materials and polymers.
Stereoselective organic synthesis
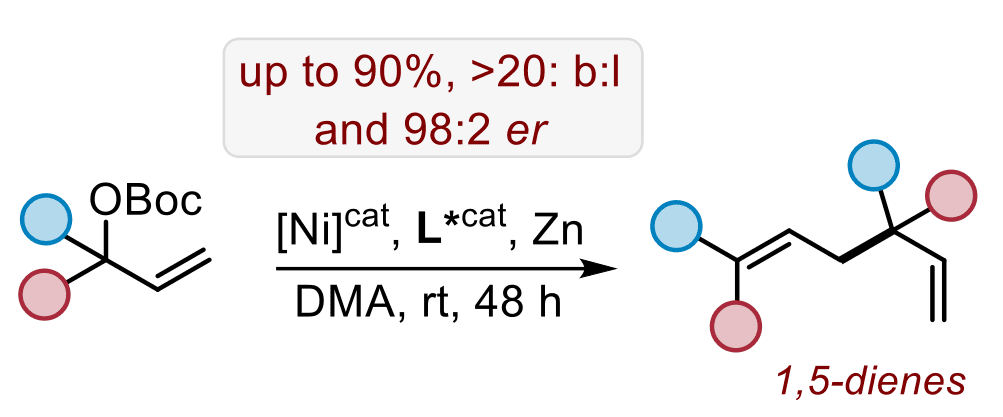
Functionalized heterocycles such as vinyl- and alkynyl-derived cyclic carbonates and carbamates are useful in the context of decarboxylative allylation and propargylation chemistry in the presence of a suitable catalyst. While we have had a great deal of success using Pd- and Cu-catalysis to afford compounds with challenging quaternary or tertiary, tetrasubstituted carbon stereocenters, more recently we switched to economically more attractive alternative catalysts derived from Co and Ni to empower similar and other types of carbon-carbon and carbon-heteroatom bond formations. For instance, a formal cross-electrophile coupling of two molecules of a tertiary (linear) carbonate affords decorated examples of 1,5-dienes that feature a quaternary stereogenic center with high levels of enantio-, diastereo-, chemo- and regio-selectivity. The experimental data was further expanded with a detailed mechanistic study allowing to conclude that the cross-coupling is mediated by both an electrophilic and a nucleophilic Ni(allyl) species with a co-catalytic role for divalent Zn.

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements

















