- Home
- Resarch
- Research Groups
- Prof. Antonio M. Echavarren
Prof. Antonio M. Echavarren
Organic synthesis and organometallic chemistry for health and material science applications
Research Overview
Prof. Echavarren research group was one of the pioneers in the field of organic chemistry employing gold catalysis. They have studied the fundamental aspects and the puzzling mechanisms of the skeletal rearrangements of enynes and other transformations that proceed through a common intermediate bearing cyclopropyl gold(I) carbenes.
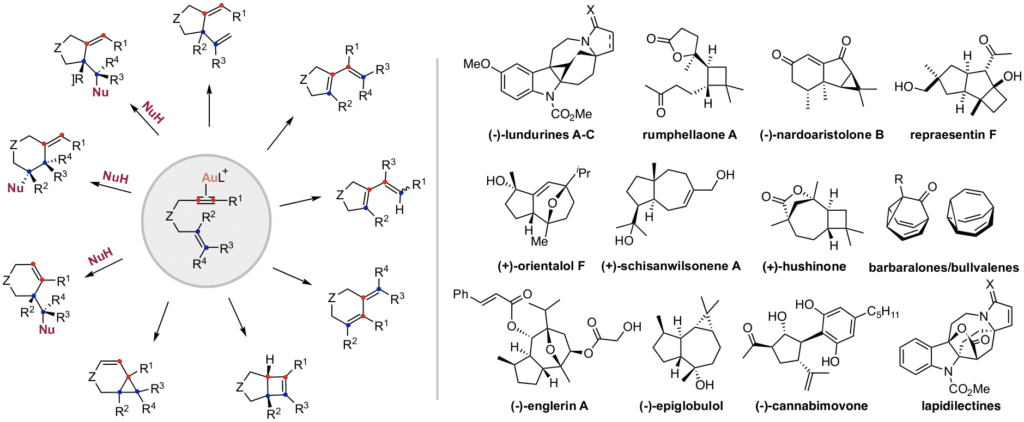 We have discovered a new reactivity of gold that can be easily applied for the total synthesis of biologically relevant natural products such as (-)-epiglobulol, cannabimovone, (–)-nardoaristolone B, repraesentin F, echinopine skeleton, lundurines, grandilodine and lapidilectine. A concise synthesis of barbaralones and bullvalenes via fluxional barbaralyl cations has been reported. Due to remarkable and unique antitumor activity of (-)-englerin A towards certain types of renal cancer, several analogues have been prepared in our group leading to the discovery of new potent compounds with broader biological profile.
We have discovered a new reactivity of gold that can be easily applied for the total synthesis of biologically relevant natural products such as (-)-epiglobulol, cannabimovone, (–)-nardoaristolone B, repraesentin F, echinopine skeleton, lundurines, grandilodine and lapidilectine. A concise synthesis of barbaralones and bullvalenes via fluxional barbaralyl cations has been reported. Due to remarkable and unique antitumor activity of (-)-englerin A towards certain types of renal cancer, several analogues have been prepared in our group leading to the discovery of new potent compounds with broader biological profile.
Following the discovery of a new method for the generation gold(I) carbenes by a catalytic retro-Buchner reaction of 7-substituted 1,3,5-cycloheptatrienes, synthetically useful reactions were developed. This method was extended for the generation of zinc carbenoids and rhodium carbenes. As mechanistic support for these studies, gold(I) carbenes generated from the corresponding carbenoids, were characterized and their involvement as genuine intermediates in catalytic transformations was demonstrated.
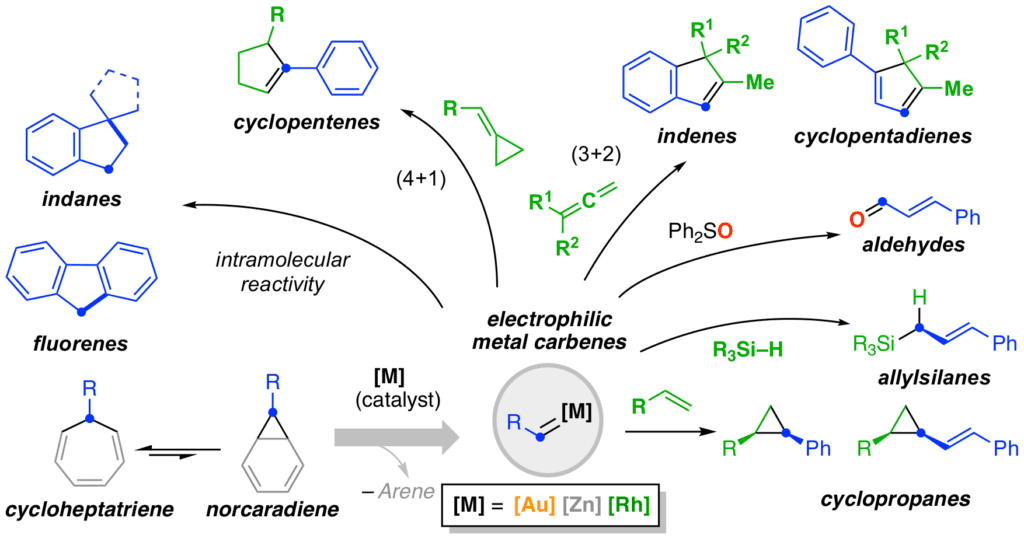
We reported the intermolecular [2+2] cycloaddition of alkynes with alkenes and applied for the synthesis of macrocycles and both rumphellaone A and hushione, natural products that contain tetrasubstituted cyclobutene moiety. The first asymmetric synthesis of cyclobutenes by intermolecular gold(I)-catalyzed [2+2] cycloaddition has been developed. More recently a catalytic system for the incorporation of acetylene gas into complex frameworks by means of gold(I) catalysis has been developed.
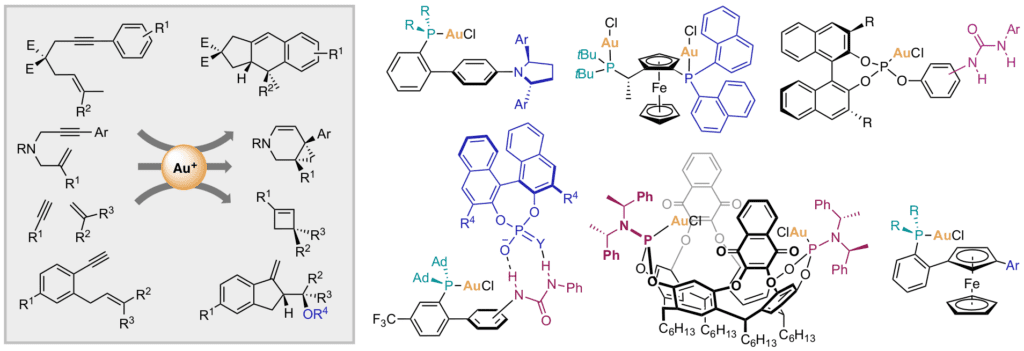
Chiral gold catalysts have been developed based on new designs for the enantioselective cyclization of unsaturated substrates. The concept of H-bonded counterion-directed enantioselective gold(I)-catalysis has been developed. As an alternative, a chiral auxiliary approach has also been developed.
We applied the gold(I)-catalyzed [4+2] cycloaddition of arylalkynes with alkenes for the ready access to stable hydroacenes, and linear acenes, such as nonacene and undecacene, by surface-metal promoted dehydrogenation. Recently, tridecacene, the longest acene with 13 rings has been prepared and characterized.
In addition, his group has developed and studied mechanistically broad-scope ruthenium, rhodium, and iridium-catalyzed Csp2–H and Csp3–H functionalization reactions.

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements
















