A new method to build complex amine-based structures using light and nickel catalysis
ICIQ researchers expand the synthetic toolbox for drug discovery and molecular design
28th March 2025 – A research team led by Prof. Ruben Martin, from the Institute of Chemical Research of Catalonia (ICIQ), has developed a new chemical method that uses light and a nickel catalyst to efficiently create complex molecular structures known as enantioenriched amine-containing sp3–sp3 architectures. These structures are important in pharmaceuticals, as drug candidates, and other chemical applications. The method starts with widely available materials (olefins and racemic aziridines) making it more practical and accessible.
The researchers developed this technique to address a common challenge in pharmaceutical chemistry: how to efficiently produce amine-containing molecules with the right 3D structure. Existing methods to achieve this require multiple steps or expensive starting materials, limiting their use. The new approach, which combines a nickel catalyst with a specially designed ligand, provides an alternative way to form these valuable molecular structures.
Relevance in modern catalysis and drug development
Reactions that form carbon-carbon (C–C) bonds between sp3-hybridised carbon atoms are highly valuable in drug discovery. In fact, many modern drugs benefit from having a greater proportion of sp3 carbons, as this improves several molecular attributes that contribute to clinical success. While chemists have developed various methods to create these bonds, there are still gaps in existing techniques, particularly when it comes to forming enantioenriched amine-containing molecules from simple and readily available starting materials.
This study, published at Nature Catalysis, provides a solution for rapidly and reliably accessing enantioenriched amines via sp3–sp3 bond-formation by merging light-induced processes and nickel catalysis. The technique makes use of both terminal and internal olefins, overcoming a major limitation of previous methods, thus holding promise for its implementation in complex molecules.
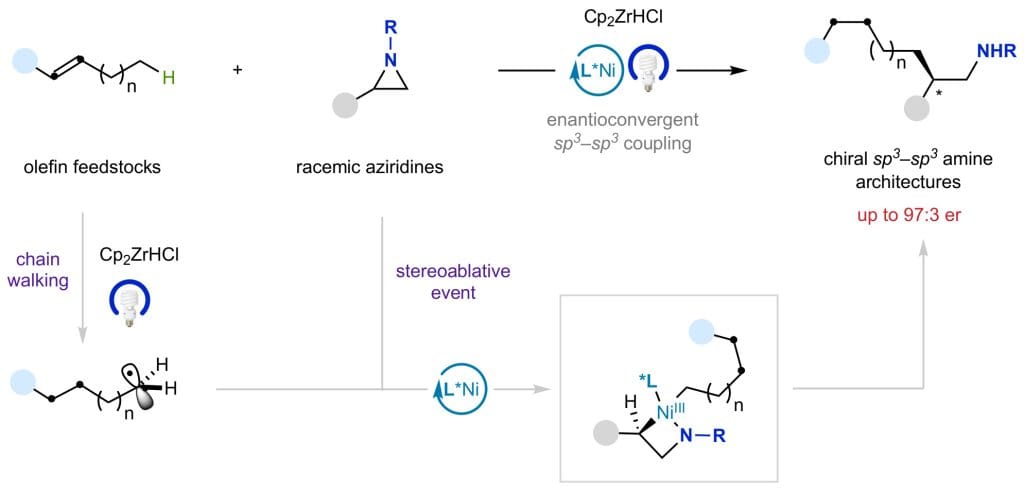
The role of aziridines
Aziridines are small, ring-shaped molecules that have long been used as building blocks in synthetic chemistry. They are particularly useful because their structure allows researchers to introduce nitrogen-containing groups while keeping the core amine functionality intact. This is an advantage in pharmaceutical applications, where amine groups are often crucial for a drug’s biological activity.
Despite their usefulness, it has been difficult to develop reactions that efficiently transform racemic aziridines into enantioenriched products while forming sp3–sp3 architectures. The method developed by Prof. Martin’s group offers new tactics in this area, expanding the potential applications of aziridines in chemical synthesis.
This newly developed method provides a practical way to create enantioenriched molecular structures with high selectivity under mild reaction conditions. The process is particularly useful for making compounds from simple olefin feedstocks, and it works even with internal olefins, where the position of the reaction is controlled by a process called chain-walking.
“We expect that the broader implications of this technique will foster systematic investigations for a more prolific use of aziridines in enantioconvergent scenarios for forging architectures of interest in medicinal chemistry settings,” stated Prof. Martin.
Reference article
Photoinduced Nickel-Catalysed Enantioconvergent sp3–sp3 Coupling of Unactivated Olefins and Aziridines
Zhang, L.; Wang, H.; Santiago, T. G.; Yue, W.-J.; Martin, R.
Nature Catalysis, 2025
10.1038/s41929-025-01319-4
Related news

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements







 21-02-2025
21-02-2025 



















