Photoinduced Nickel-Catalysed Enantioconvergent sp3–sp3 Coupling of Unactivated Olefins and Aziridines
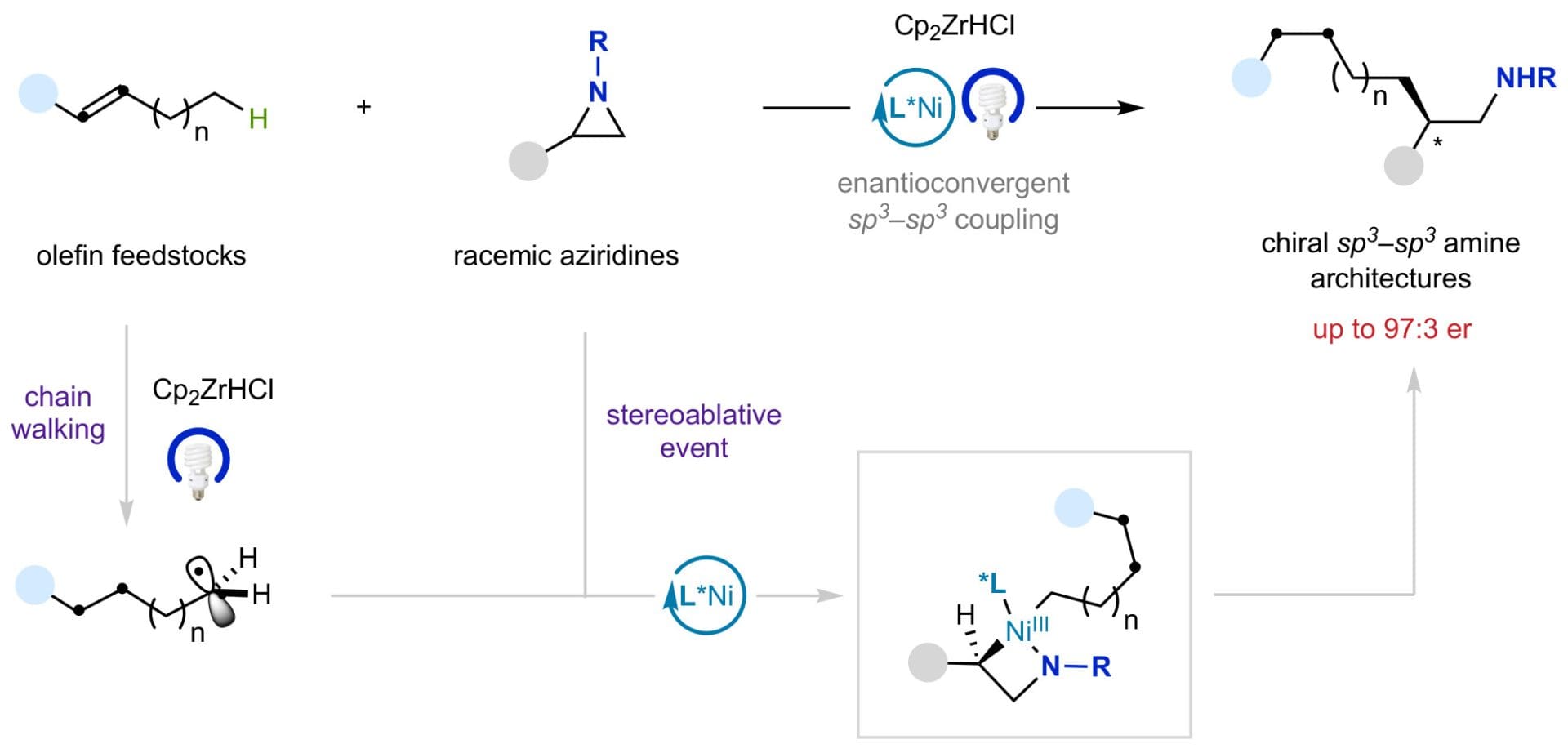
Catalytic sp3–sp3 bond-forming reactions have found considerable echo in both academic and pharmaceutical laboratories. This is largely due to the observation that a higher content in sp3
hybridized carbons has recently shown to improve several molecular attributes that contribute to clinical success. Although the ready availability of unactivated olefins and aziridines makes them ideal precursors to forge enantioenriched sp3–sp3 architectures with added-value amine functions, an enantioconvergent catalytic scenario of these counterparts has not yet been realized. Herein, we describe a nickel-catalysed blueprint that enables the enantioselective construction of aminecontaining sp3–sp3 architectures via photoinduced enantioconvergent coupling of racemic aziridines with alkyl zirconium reagents generated in situ from unactivated terminal and even internal olefins. The broadly applicability of this protocol is illustrated in a series of late-stage diversification of advanced synthetic intermediates.
Zhang, L.; Wang, H.; Santiago, T. G.; Yue, W.-J.; Martin, R.
Nat. Catal. 2025
DOI:
10.1038/s41929-025-01319-4

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements




















