ICIQ – Crysforma
Pharmaceutical solid state services


Crysforma provides complete scientific support for the discovery, analysis and scale-up of polymorphs, hydrates, amorphous phases, salts and co-crystals of active pharmaceutical ingredients or intermediates.
The main working areas are:
- The modification of the solid state properties of active compounds to obtain the most suitable for the desired application (polymorphism, salts and co-crystal screenings).
- The optimisation of crystallisation processes to improve the development stages and to obtain the solids with the desired properties (purity, particle size, polymorph, morphology,…).
- The characterisation of the solid state of drug substance and drug product (PXRD, SCXRD, microED, DSC, TGA, DVS, PSD, IR, Raman, NMR, synchrotron…).
- Supporting the IP protection of new generated solid forms.
Crysforma has developed its own crystallisation screening methodology based on the combination of several crystallisation techniques and the individual monitoring of the experiments by highly skilled scientists to maximise the information extracted from each crystallisation. Our projects are always carried out in close collaboration with the client, adapted to the customer’s needs and under strict confidentiality conditions.
Expertise
Equipment
-
Dynamic Vapor Sorption
Determine the effects of relative humidity and temperature on the solid form of interest in combination with a climatic chamber and the powder X-ray diffraction analysis at variable relative humidity.
-
Electron diffractometer

Elucidation of the structure and determination of the absolute configuration with a very small amount of material and “nano” crystals.
-
Particle size distribution
Development and validation of analytical methods to determine the PSD of samples using a laser diffraction methodology (wet dispersion).
-
Powder X-Ray diffractometer
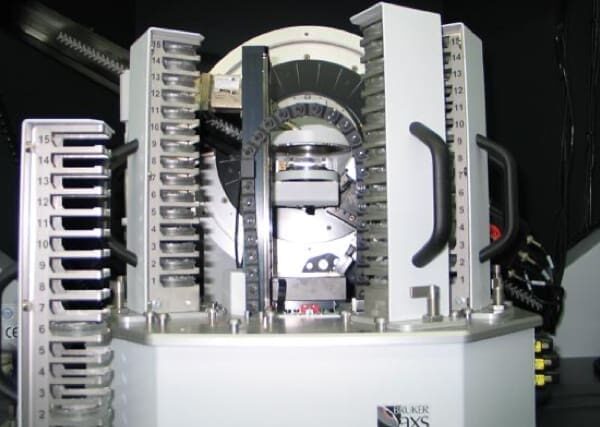
Crystalline form quantification and detection limit determination. Fast analysis service. Variable humidity and temperature analysis. Analysis of drug substance and drug product.
-
Single crystal X-Ray diffractometer
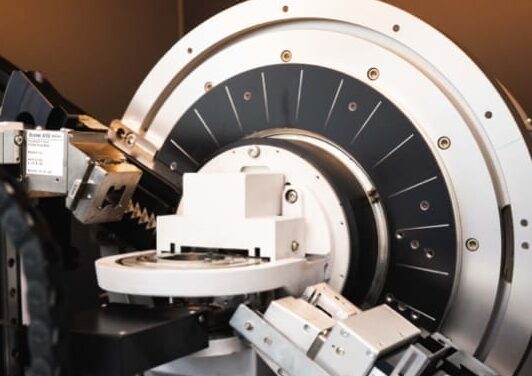
Elucidation of the structure and determination of the absolute configuration. Crystallisation screenings to grow up suitable single crystal for analysis.
-
Thermal analysis

Differential scanning calorimetry, thermogravimetric analysis, hot-stage microscopy to characterise the thermal process of a given sample.
Events
-
April 2025
30
ExhibitionSant Jordi's Day Celebration at ICIQ (2025)
-
May 2025
16
SeminarApproaches and Catalyst’s Designs in Anion-Binding and Acridinium Photocatalysis
-
June 2025
10
SeminarSynthetic Applications and Mechanistic Considerations of Nickel- and Photo-Catalysis
-
June 2025
27
SeminarExploiting Host-Guest Chemistry in Sensing Array, Enrichment of PTMs on Histone Protein and RNA, and Water Micropollutant Detection and Removal

Get started with an expert
Together, let’s create a brighter future providing solutions through a partnership
Connect with us























