Trifluoromethylation of α‑haloketones highlighted by Synfacts
If last month Synfacts selected Prof. Grushin et al. method for the trifluoromethylation of aryl boronic acids, this month they highlight the first nucleophilic trifluoromoethylation of ?-haloketones published by the same research group in JACS.
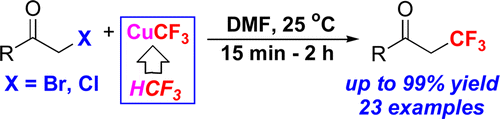
Graphical Abstract
This new method requires low-cost chemicals to afford the corresponding trifluoromethylated products with up to 99% yield, at room temeprature and under short reaction times. Thereby, this novel synthetic procedure is an unprecedentedly easy entry to valuable 2,2,2-trifluoroethylketones.
The Editorial Board of SYNFACTS considered this paper to be one of the most significant recent developments and future trends in synthetic chemistry.
Related news

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements






 27-05-2022
27-05-2022 


















