The surprises of scaling up
Following up on a 2017 JACS paper, researchers from the Muñiz group together with CSOL, ICIQ’s technology development Unit, set out to scale up the synthesis of enantiopure aryl-iodine pre-catalysts. After some surprising discoveries, the scientists have published an improved and upscaled reaction in Organic Letters, where they describe how to produce up to 20 grams of the pre-catalyst in a third of the original time.
The pre-catalyst is used to form chiral diamines with relevance for organic synthesis. Many 1,2-diamines have biological activity and thus have significance for the pharmaceutical industry, exemplified by drugs like the antiparasitic levamisole or the antidepressant mirtazapine. Reaction selectivity is important for all chemists, but it’s actually a major issue for the pharma industry. A large percentage of drug syntheses yield racemic mixtures – which have equal amounts of the possible enantiomers of a chiral molecule. Separating these mixtures into their respective enantiomers takes extra time, money, energy, and generates waste. Thus, it is of great importance to find selective routes to produce the desired enantiomer – at an industrial scale.
This collaboration between CSOL and the Muñiz group was enthusiastically undertaken by late Prof. Kilian Muñiz, to whom the paper is dedicated. The research has received funding from the Spanish Science and Innovation Ministry FEDER grant (CTQ2017-88496R), and valorisation funds donated by ”la Caixa” Foundation.
“Our main goal was to make this catalyst more accessible to the community,” explains Èric Cots, PhD student in the Muñiz group and first author of the paper. “The original lab-scale synthesis was 60-hour long and up to two grams, but the improved reaction takes only a third of that and we can make up to 20 grams of the pre-catalyst, avoiding all cumbersome column chromatographies.” But time isn’t the only improvement the researchers have made to the reaction.
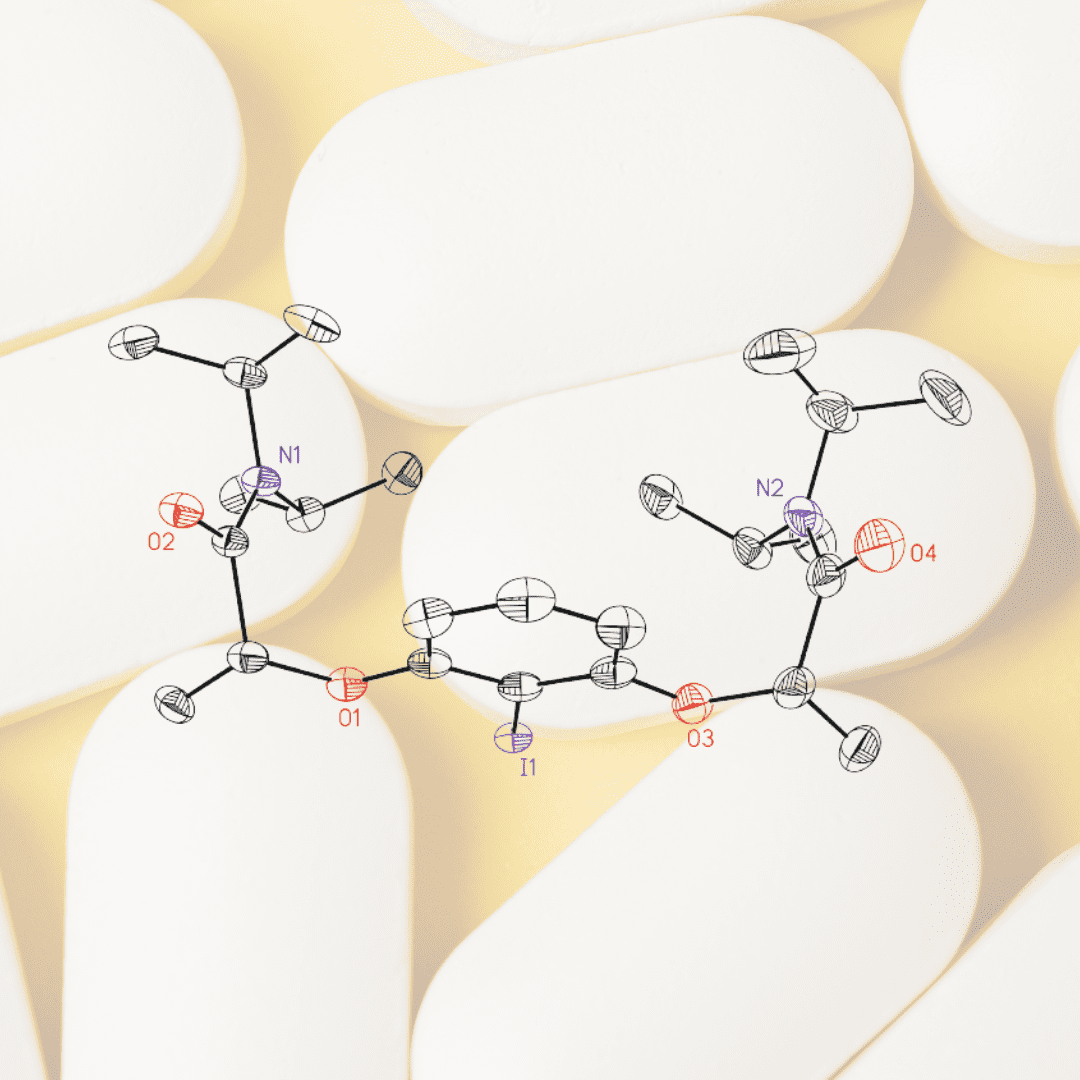
After the first scaling-up, hoping to prove that the upscaled reaction provided the catalyst with the same properties as the lab-scale reaction, the researchers tested the catalyst in the previously reported diamination reaction. To their dismay, the reaction yielded racemic products. A thorough examination of the samples from each step of the synthesis indicated that the meso form of the catalysts was formed due to the basic conditions employed in the last step. This meso form was very likely also formed in the two gram-scale reaction but went unnoticed, so it has been the scale-up that has surfaced this previously unknown issue.
The problem was solved by a small modification of the synthetic procedure and the catalysts were successfully synthesised with complete avoidance of contamination by the meso form. This optimised procedure was tested in several diamination reactions, providing exquisite levels of enantioselectivity, higher than the previously reported results. “For all the products we tested, we improved their enantioselectivity. It is the first time this reaction reaches these levels of enantiomeric excess – which would be useful to the pharmaceutical industry in the future” states Cots.
The team hopes that the scaled process will have an impact on the science of iodine I/III catalysis. “This should facilitate pharma’s access to enantiopure chiral diamines,” explains Dr. Fernando Bravo, CSOL Unit manager and main author of the paper. “The more and better options the pharmaceutical industry has, the easier it will be to substitute poisonous heavy metals (particularly palladium) that are currently used on these kinds of transformations.”
Reference article:
Deciphering the Keys for High Enantioselectivity in Hypervalent Iodine-Catalyzed 1,2-Difunctionalization: Improved Synthesis of Ishihara–Muñiz Precatalysts. Cots, E.; Rintjema, J.; Bravo, F.; Muñiz, K.; Org. Lett. 2021, DOI: 10.1021/acs.orglett.1c02252.
Related news

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements






 11-12-2024
11-12-2024