Synfacts selects Grushin et al. method for the trifluoromethylation of aryl boronic acids
The new method for the trifluoromethylation of aryl boronic acids developed by Petr Novák, Anton Lishchynskyi and Vladimir Grushin and published in Angew. Chem. Int. Ed. last June, has now been selected by the Editorial Board of SYNFACTS for its important insights.
The paper describes the first trifluoromethylation of aryl-boronic acids with low-cost fluoroform-derived CuCF3.
The reaction occurs at room temperature, exhibits unprecedented functional-group tolerance and affords trifluoromethylated aromatic compounds in up to 99 % yield.
The reaction occurs at room temperature, exhibits unprecedented functional-group tolerance and affords trifluoromethylated aromatic compounds in up to 99 % yield.
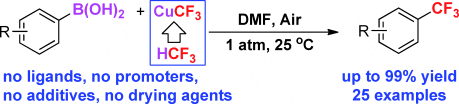
This paper was recently highlighted by Chemistry Views with an article intitled ‘Low Cost, Simple, Efficient, Safe Trifluoromethylation’.
Petr Novák, Anton Lishchynskyi, Vladimir V. Grushin,
Angew. Chem. Int. Ed. 2012, 51, 7767–7770.
Related news

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements






 27-05-2022
27-05-2022 


















