Synfacts highlights a paper by Melchiorre's group
A paper published by Prof. Melchiorre research group in Journal of the American Chemical Society has been highlighted in Synfacts, Vol 11, Issue 7, 765. Synfacts selects the most significants results on synthetic organic chemistry that appear in the literature considered as the future trends in synthetic chemistry.
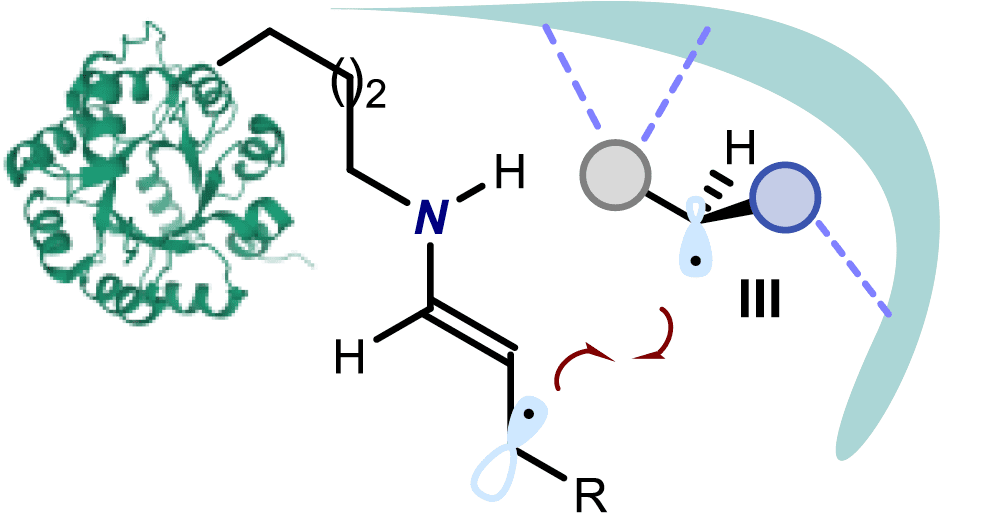
The paper deals with a photo-organocatalytic enantioselective α- and γ-alkylation of aldehydes and enals, respectively, with bromomalonates. The chemistry uses a commercially available aminocatalyst and occurs under illumination by a fluorescent light bulb in the absence of any external photoredox catalyst. Mechanistic investigations reveal the previously hidden ability of transiently generated enamines to directly reach an electronically excited state upon light absorption while successively triggering the formation of reactive radical species from the organic halides. At the same time, the ground state chiral enamines provide effective stereochemical induction for the enantioselective alkylation process.
Enantioselective Organocatalytic Alkylation of Aldehydes and Enals Driven by the Direct Photoexcitation of Enamines
M. Silvi, E. Arceo, I. D. Jurberg, C. Cassani, P. Melchiorre
J. Am. Chem. Soc., 2015, 137, 6120-6123
Related news

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements







 22-11-2024
22-11-2024