Water oxidation at a tetraruthenate core stabilized by polyoxometalate ligands: Experimental and computational evidence to trace the competent intermediates
Converging UV-vis, EPR, rRaman, and DFT calculations highlight the evolution of [Ru4(H2O)4(μ-O)4(μ-OH)2(γ-SiW10O36)2]10-, 1, to high-valent intermediates. In analogy with the natural enzyme, five different oxidation states, generated from 1, have been found to power the catalytic cycle for water oxidation. A high electrophilic tetraruthenium(V)-hydroxo species is envisaged as the competent intermediate, undergoing nucleophilic attack by an external water molecule as a key step in the formation of a new O-O bond under catalytic conditions.
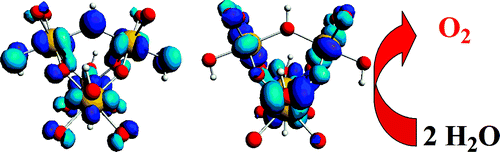
A. Sartorel, P. Miró, E. Salvadori, S. Romain, M. Carraro, G. Scorrano, M. Di Valentin, A. Llobet, C. Bo, M. Bonchio
J. Am. Chem. Soc. 2009, 131, 16051-16053
Associated ICIQ research group/s:

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements




















