Unraveling the Role of Particle Size and Nanostructuring on the Oxygen Evolution Activity of Fe-Doped NiO
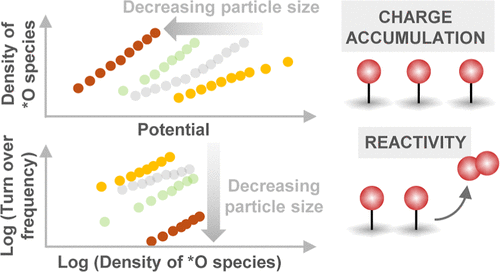
Nickel-based oxides and oxyhydroxide catalysts exhibit state-of-the-art activity for the sluggish oxygen evolution reaction (OER) under alkaline conditions. A widely employed strategy to increase the gravimetric activity of the catalyst is to increase the active surface area via nanostructuring or decrease the particle size. However, the fundamental understanding about how tuning these parameters influences the density of oxidized species and their reaction kinetics remains unclear. Here, we use solution combustion synthesis, a low-cost and scalable approach, to synthesize a series of Fe0.1Ni0.9O samples from different precursor salts. Based on the precursor salt, the nanoparticle size can be changed significantly from similar to 2.5 to similar to 37 nm. The OER activity at pH 13 trends inversely with the particle size. Using operando time-resolved optical spectroscopy, we quantify the density of oxidized species as a function of potential and demonstrate that the OER kinetics exhibits a second-order dependence on the density of these species, suggesting that the OER mechanism relies on O-O coupling between neighboring oxidized species. With the decreasing particle size, the density of species accumulated is found to increase, and their intrinsic reactivity for the OER is found to decrease, attributed to the stronger binding of *O species (i.e., a cathodic shift of species energetics). This signifies that the high apparent OER activity per geometric area of the smaller nanoparticles is driven by their ability to accumulate a larger density of oxidized species. This study not only experimentally disentangles the influence of the density of oxidized species and intrinsic kinetics on the overall rate of the OER but also highlights the importance of tuning these parameters independently to develop more active OER catalysts.
Rao, R. R.; Bucci, A.; Corby, S.; Moss, B.; Liang, C.W.; Gopakumar, A.; Stephens, I. E. L.; Lloret-Fillol, J.; Durrant, J. R.
ACS Catal. 2024,
DOI:
10.1021/acscatal.4c02329

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















