The Role of Seven Coordination in Ru-catalyzed Water Oxidation
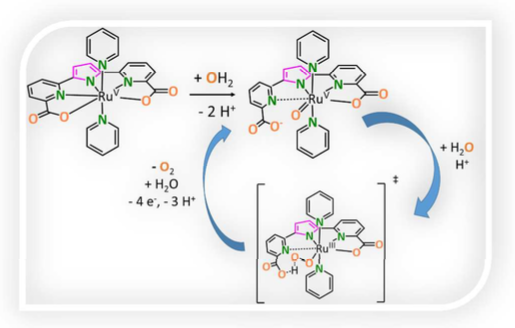
A family of Ru complexes based on the t5a3- ligand ((2,5-bis(6-carboxylatopyridin-2-yl)pyrrol-1-ide) and pyridine (py) that include, {RuII(Ht5a--N2O)(py)3}, 1HII(-N2O), {RuIII(t5a--N3O1.5)(py)2}, 2III(-N3O1.5) and {RuIV(t5a--N3O2)(py)2}, 2IV(-N3O2) has been prepared and thoroughly characterized. Complexes 1HII(-N2O), 2III(-N3O1.5) and 2IV(-N3O2) have been investigated in solution by spectroscopic methods (NMR, UV-vis) and in the solid state by single-crystal X-ray diffraction analysis and complemented by density functional theory (DFT) calculations. The redox properties of complex 2III(-N3O1.5) have been investigated by electrochemical methods (CV and DPV), showing its easy access to high oxidation states, thanks to the trianionic nature of the t5a3- ligand. In neutral to basic conditions complex 2IV(-N3O2) undergoes aquation generating {RuIV(OH)(t5a--N2O)(py)2}, 2IV(OH)(-N2O). Further oxidation of the complex forms {RuV(O)(t5a--N2O)(py)2}, 2V(O)(-N2O) that is a very efficient water oxidation catalysts, reaching TOFMAX of 9400 s-1, as measured via foot of the wave analysis. The key to fast kinetics for the catalytic oxidation of water to dioxygen by 2V(O)(-N2O) is due to not only the easy access to high oxidation states but also to the intramolecular hydrogen bonding provided by the non-coordinated dangling carboxylate at the transition state, as corroborated by DFT calculations.
R. Matheu, M.Z. Ertem, M. Pipelier, J. Lebreton, D. Dubreuil, J. Benet-Buchholz, X. Sala, A. Tessier, A. Llobet
ACS Catal. 2018, 8, 2039-2048
DOI:
10.1021/acscatal.7b03638

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















