The role of manganese in CoMnOx catalysts for selective long-chain hydrocarbon production via Fischer-Tropsch synthesis
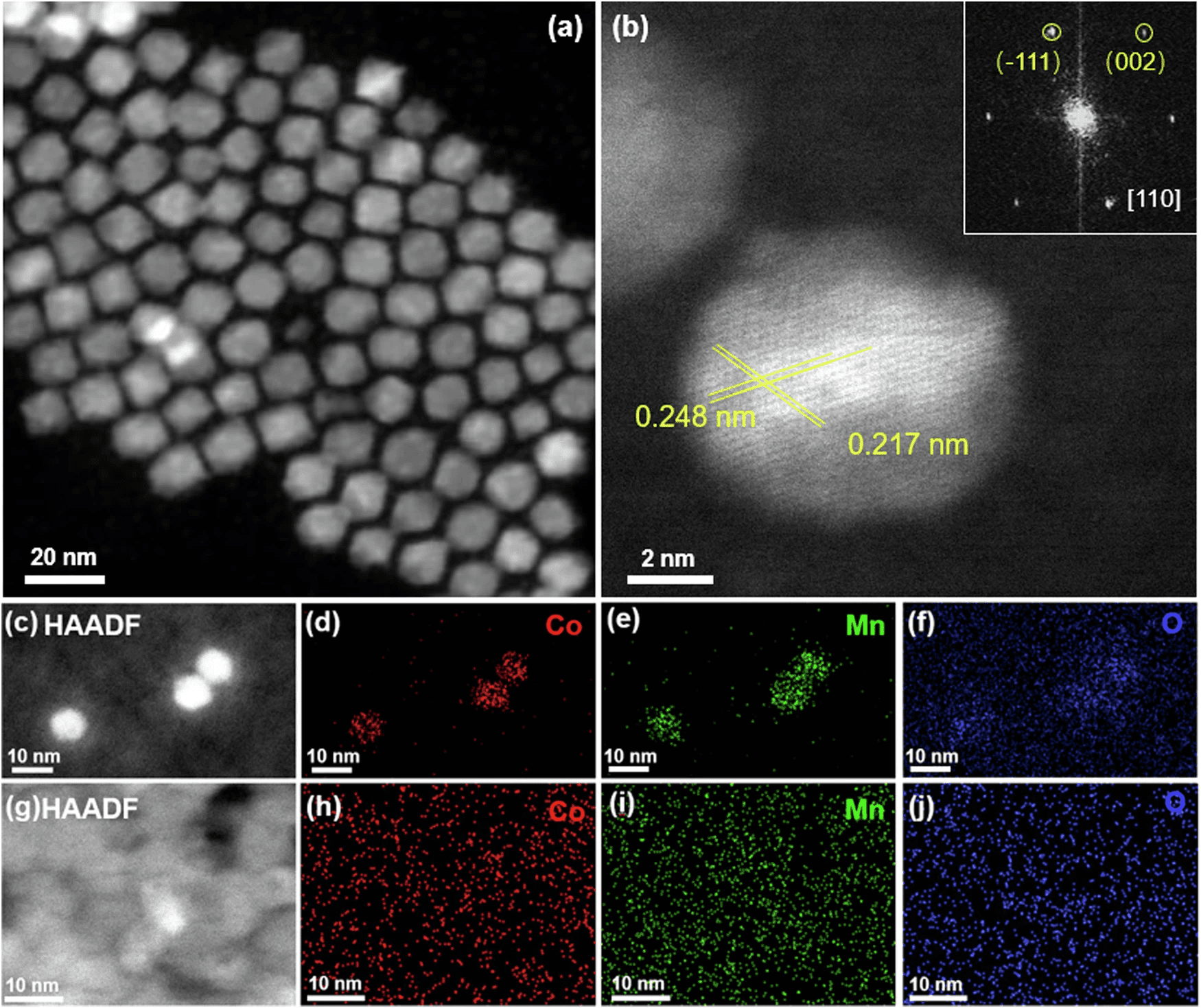
Cobalt is an efficient catalyst for Fischer−Tropsch synthesis (FTS) of hydrocarbons from syngas (CO + H2) with enhanced selectivity for long-chain hydrocarbons when promoted by Manganese. However, the molecular scale origin of the enhancement remains unclear. Here we present an experimental and theoretical study using model catalysts consisting of crystalline CoMnOx nanoparticles and thin films, where Co and Mn are mixed at the sub-nm scale. Employing TEM and in-situ X-ray spectroscopies (XRD, APXPS, and XAS), we determine the catalyst’s atomic structure, chemical state, reactive species, and their evolution under FTS conditions. We show the concentration of CHx, the key intermediates, increases rapidly on CoMnOx, while no increase occurs without Mn. DFT simulations reveal that basic O sites in CoMnOx bind hydrogen atoms resulting from H2 dissociation on Co0 sites, making them less available to react with CHx intermediates, thus hindering chain termination reactions, which promotes the formation of long-chain hydrocarbons.
Chen, H.; Lian, Z.; Zhao, X.; Wan, J.; Pieters, P.F.; Oliver-Meseguer, J.; Yang, J.; Pach, E.; Carenco, S.; Treps, L.; Liakakos, N.; Shan, Y.; Altoe, V.; Wong, E.; Zhuo, Z.; Yang, F.; Su, J.; Guo, J.; Blum, M.; Lapidus, S.H.; Hunt, A.; Waluyo, I.; Ogasawara, H.; Zheng, H.; Yang, P.; Bell, A.T.; López, N.; Salmeron, M.
Nat. Commun. 2024, 15, 10294
DOI:
10.1038/s41467-024-54578-3

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















