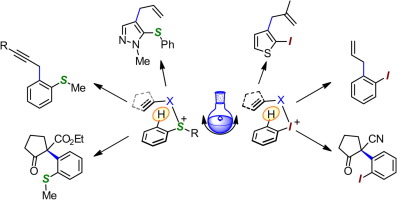
The emergence of sulfoxide and iodonio-based redox arylation as a synthetic tool (Review-Digest)
This digest highlights the emergence of the directed metal-free arylation approach employing simple sulfoxide and hypervalent aryliodane. These new processes are characterized by an unusual, and synthetically attractive, retention of the reduced −SR and iodine fragment ortho to the newly formed C
A Shafir
Tetrahedron Letters 2016, 57 (25), 2673-2682
DOI:
Go to the journal

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements