The effect of solvent on the binding of anions and ion-pairs with a neutral [2]rotaxane
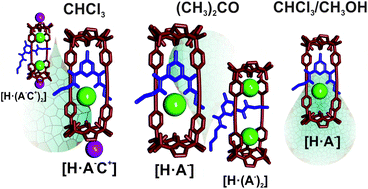
In this work we report the binding properties of rotaxane 1 towards a series of tetraalkylammonium salts of Cl−, OCN− and NO3− anions in acetone and a CHCl3/MeOH solvent mixture. We used 1H NMR titrations and Isothermal Titration Calorimetry (ITC) experiments to monitor and analyze the binding processes. We compared the obtained results with those previously described by us in chloroform solution. In acetone solution, the determined binding constants for the 1 : 1 complexes were 1 to 3 orders of magnitude larger than those measured in chloroform, a less competitive solvent for hydrogen-bonding. The thermodynamic signatures of the binding processes in acetone, determined by ITC experiments, revealed favorable enthalpic and entropic contributions having similar magnitudes. These results suggested that solvation/desolvation processes in acetone play a significant role in the binding processes. Conversely, the addition of just 5% of methanol to chloroform solutions of 1 significantly reduces the magnitude of the binding constants of all studied ion-pairs. In this solvent mixture, the entropy term is also favorable but it does not compensate the experienced loss of binding enthalpy. Moreover, in acetone solution, the addition of the Cl− and OCN− tetraalkylammonium salts in excess (more than 1 equiv.) led to the immediate appearance of 2 : 1 complexes. Related high-stoichiometry complexes are not observed in the solvent mixture (CHCl3/MeOH 95/5). In chloroform, a large excess of the salt (> 6 equiv.) is required for its formation. From the analysis of the obtained binding data we infer that, in acetone and in CHCl3/MeOH mixture, the formed complexes are mainly anionic.
Molina-Muriel, R.; Romero, J. R.; Li, Y.; Aragay, G.; Ballester, P.
Org. Biomol. Chem. 2021, 19, 9986-9995
DOI:
10.1039/D1OB01845K

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements




















