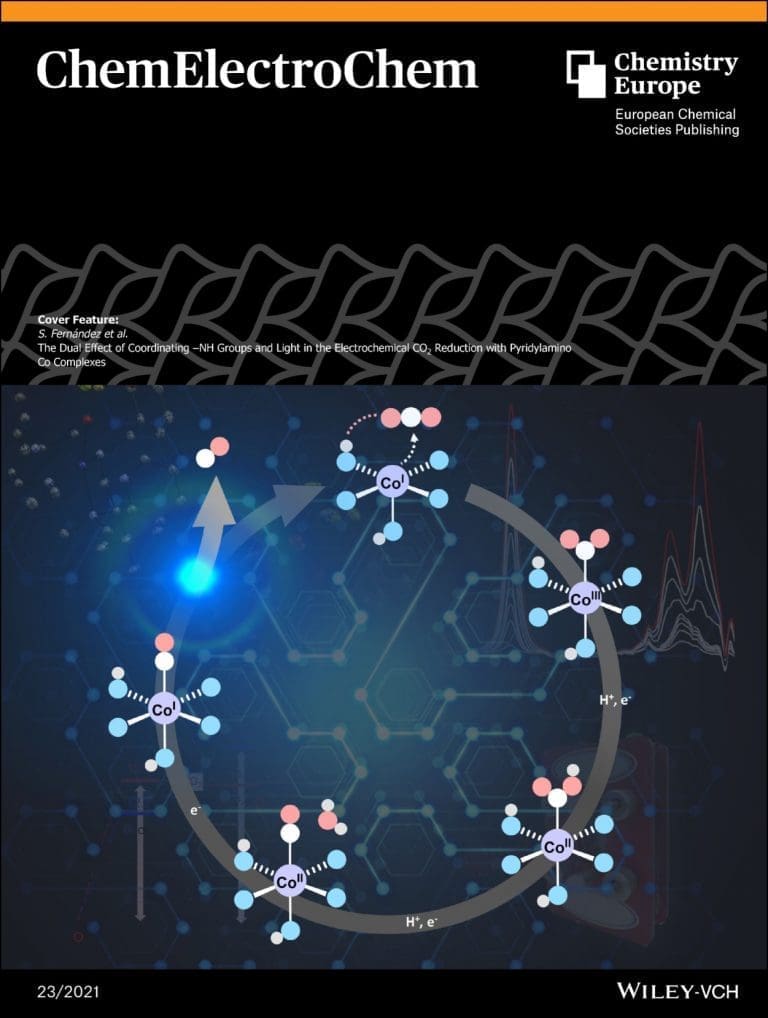
The dual effect of coordinating –NH groups and light in the electrochemical CO2 reduction with pyridylamino Co complexes
CO2 electroreduction could be improved by applying conceptualized strategies to overcome catalytic bottlenecks. In this regard, we report two new cobalt(II) complexes [Co(Py2R N3)(OTf)](OTf) (CoR , R = H, Me) based on a new C2 -symmetric pentacoordinate chiral ligand that are active on the electrochemical CO 2 reduction to CO. One of the complexes has a N–H group oriented towards the CO2 binding site (CoH ), while the other has a N–Me group with the same orientation (CoMe ), as showed by X-ray diffraction. We have studied the effect of introducing hydrogen bonding sites, i.e. N–H in Co H , as a strategy to stabilize reaction intermediates. The complex bearing coordinating unprotected N–H group (Co H ) displays catalytic CO2 reduction at the CoII/I redox potential (-1.9 V vs. Fc, ca. 40% FYCO). Whereas CoMe shows CO2 reduction at the CoI/0 redox pair. FTIR-SEC and DFT calculations allowed for the identification of a [CoI-CO]+ cation as a catalytic intermediate. The beneficial effect of the N–H group has been attributed to the stabilization of reaction intermediates or transition states and by the larger electron-donating capacity enhancing the nucleophilic character of the CoI intermediate. The study also points to the CO dissociation from the Co(I)-CO resting state intermediate as one of the bottlenecks of the catalytic cycle, which can be overcome with light irradiation, resulting in an increase of the total CO production (-1.9 V, 81% FYCO, 11 TONCO) at the CoII/I potential.

Lloret Fernández, S.; Cañellas, S.; Franco, F.; Luis, J. M.; Pericàs, M. A.; Lloret-Fillol, J.
ChemElectroChem 2021, 8 (23), 4456-4465
DOI:
10.1002/celc.202100859

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements