Tandem electrocatalytic CO2 reduction with Fe-porphyrins and Cu nanocubes enhances ethylene production
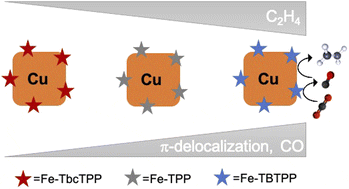
Copper-based tandem schemes have emerged as promising strategies to promote the formation of multi-carbon products in the electrocatalytic CO2 reduction reaction. In such approaches, the CO-generating component of the tandem catalyst increases the local concentration of CO and thereby enhances the intrinsic carbon–carbon (C–C) coupling on copper. However, the optimal characteristics of the CO-generating catalyst for maximizing the C2 production are currently unknown. In this work, we developed tunable tandem catalysts comprising iron porphyrin (Fe-Por), as the CO-generating component, and Cu nanocubes (Cucub) to understand how the turnover frequency for CO (TOFCO) of the molecular catalysts impacts the C–C coupling on the Cu surface. First, we tuned the TOFCO of the Fe-Por by varying the number of orbitals involved in the π–system. Then, we coupled these molecular catalysts with the Cucub and assessed the current densities and faradaic efficiencies. We discovered that all of the designed Fe-Por boost ethylene production. The most efficient Cucub/Fe-Por tandem catalyst was the one including the Fe-Por with the highest TOFCO and exhibited a nearly 22-fold increase in the ethylene selectivity and 100 mV positive shift of the onset potential with respect to the pristine Cucub. These results reveal that coupling the TOFCO tunability of molecular catalysts with copper nanocatalysts opens up new possibilities towards the development of Cu-based catalysts with enhanced selectivity for multi-carbon product generation at low overpotential.
Wang, M.; Nikolaou, V.; Loiudice, A.; Sharp, I. D.; Llobet, A.; Buonsanti, R.
Chem. Sci. 2022, (13), 12673-12680
DOI:
10.1039/d2sc04794b

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements




















