Stability, reutilization, and scalability of activated hydrotalcites in aldol condensation
A number of studies have shown that solid bases, among others activated hydrotalcites, are highly efficient catalysts for C-C bond formation reactions. A widely studied case is the aldol condensation of citral and acetone, where rehydrated Mg-Al hydrotalcite shows higher yields to pseudoionone compared to NaOH solutions. Despite this fact, the fine chemical industry still operates with the traditional liquid inorganic bases. This manuscript addresses technical aspects that can explain the limited implementation of activated hydrotalcites in aldol condensations. For this purpose, the catalyst stability in air, its reusability and regenerability after reaction, and the process scalability were investigated. The conciliation of activity data of the fresh hydrotalcite in batch laboratory (ml) and bench (l) reactors with on line ATR analysis is excellent, revealing that the citral-acetone reaction over hydrotalcite can be upscaled. However, the poisoning of the active Brønsted basic centers in the rehydrated hydrotalcite by CO2 is very fast (50% activity loss after 1 h exposure to ambient). Besides, the catalyst after one run is inactive due to the presence of strongly adsorbed products and requires time-consuming (and not fully complete) regeneration. Basic centers of Lewis nature in calcined hydrotalcites (basically MgO) are more stable, but their activity is very low compared to the rehydrated counterpart. Both the technical disadvantages of current solid bases and the lack of stringent environmental regulations motivate the conservatism of industry to use alkaline solutions.
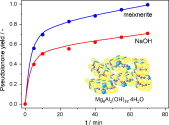
S. Abelló, D. Vijaya-Shankar, J. Pérez-Ramírez
Appl. Catal. A-Gen. 2008, 342, 119-125

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















