Selective binding of nitrate by a neutral bis(calix[4]pyrrole) [2]rotaxane
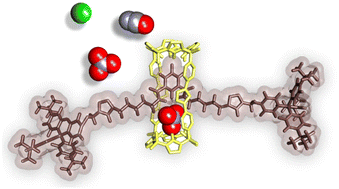
We describe the synthesis and characterization of a [2]rotaxane based on a bis calix[4]pyrrole (C[4]P) macrocycle containing two different two-walls aryl-extended C[4]P binding sites connected by a triazole spacer and a 3,5-bis-amidopyridyl-N-oxide derivative as a linear component. Our study focuses on the binding properties of the interlocked receptor towards chloride, cyanate, and nitrate tetraalkylammonium salts. We use 1H NMR spectroscopy and isothermal titration calorimetry (ITC) experiments to monitor the formation and thermodynamically characterize the complexes, respectively. The results show that adding 1 equiv. of the tetraalkylammonium salts (ion-pairs) to a millimolar receptor solution produces the quantitative assembly of two 1 : 1 isomeric inclusion complexes. The isomeric complexes differ in the anion’s and axle’s relative locations concerning the two chemically non-equivalent C[4]P hemispheres of the macrocyclic component. The interlocked receptor shows a remarkable selectivity for nitrate over chloride and cyanate binding in acetone and chloroform solutions. Ion-paired complexes are mainly formed in chloroform solution, while the anionic counterparts are prevalent in the more polar acetone solvent. We also compare the binding properties exhibited by the interlocked receptor with those previously reported for a [2]rotaxane analog featuring a slightly larger macrocyclic component and two identical binding sites. Remarkable selectivity for nitrate by a neutral [2]rotaxane based on a bis(calix[4]pyrrole) macrocycle.
Li, Y.; Molina-Muriel, R.; Aragay, G.; Ballester, P.
Org. Chem. Front. 2024,
DOI:
10.1039/d4qo01190b

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















