Rhodium-Catalysed ortho-Alkynylation of Nitroarenes
The ortho-alkynylation of nitro-(hetero)arenes takes place in the presence of a Rh(III) catalyst to deliver a wide variety of alkynylated nitroarenes regioselectively. These interesting products could be further derivatized by selective reduction of the nitro group or palladium-catalysed couplings. Experimental and computational mechanistic studies demonstrate that the reaction proceeds via a turnover-limiting electrophilic C–H metalation ortho to the strongly electron-withdrawing nitro group.
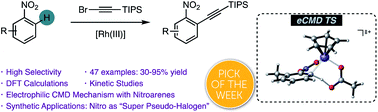
Tan, E.; Montesinos-Magraner, M.; García-Morales, C.; Guillem Mayans, J.; Echavarren, A. M.
Chem. Sci. 2021, 12, 14731-14739
DOI:
10.1039/D1SC04527J

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements




















