Removing the superfluous: a supported squaramide catalyst with a minimalistic linker applied to the enantioselective flow synthesis of pyranonaphthoquinones
A continuous flow setup has been implemented for the enantioselective production of a library of pyranonaphthoquinones. This was possible through a sequential two-step process involving a squaramide-catalyzed Michael reaction and oxa-Michael cyclization. A key factor for the success of this methodology was the development of a new, cost-effective polystyrene-supported squaramide.
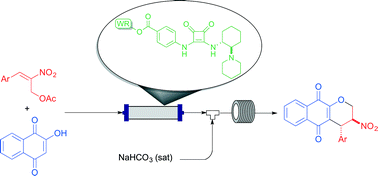
L. Osorio-Planes, C. Rodríguez-Escrich, M. A. Pericàs
Catal. Sci. Technol. 2016, 6, 4686-4689
DOI:
Go to the journal

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















