Reconstruction of dawsonite by alumina carbonation in (NH4)2CO3: Requisites and mechanism
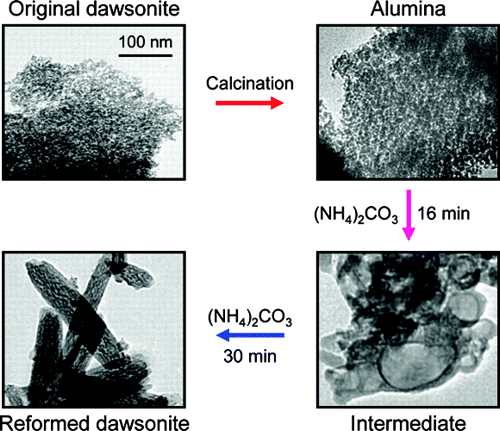
The products derived from thermal decomposition of NH4, K, and Na dawsonites have structural memory; that is, the original mineral structure in ammonium form (NH4AlCO3(OH)2) is recovered upon treatment of the oxide in aqueous (NH4)2CO3 solution under mild conditions at pH ~ 10. The memory effect holds in aluminas doped with transition metals such as chromium or iron. In contrast, treatment of calcined dawsonites in K2CO3 and Na2CO3 solutions leads to bayerite. The mechanism and kinetics of the reconstruction process were investigated by experiments in a parallel-reactor system varying treatment time, temperature, molar (NH4)2CO3/Al2O3 ratio, (NH4)2CO3 concentration, solvent, and dawsonite composition. The samples at different stages of the treatment in ammonium carbonate were characterized by X-ray diffraction, infrared spectroscopy, transmission and scanning electron microscopies, nitrogen adsorption, mercury intrusion porosimetry, and thermogravimetry. The reconstruction of the dawsonite structure from alumina follows a dissolution-precipitation mechanism and is accomplished in 10-30 min depending on the temperature. The transformation goes through an intermediate carbonate-containing aluminum hydroxide compound of amorphous nature followed by progressive dawsonite crystallization upon ammonium incorporation. In contrast with other families of materials having structural memory such as hydrotalcites, the original and reconstructed dawsonites present marked morphological and porosity differences. Upon reconstruction, nanoparticles in the as-made and calcined materials gradually transformed by way of complex intermediate morphologies into acicular nanoneedles with newly developed microporosity. The facile carbonation of alumina-related compounds in ammonium carbonate is potentially applicable for CO2 mineralization.
G. Stoica, J. C. Groen, S. Abelló, R. Manchanda, J. Pérez-Ramírez
Chem. Mater. 2008, 20, 3973-3982

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















