Organocatalytic N-formylation of amines by CO2 in batch and continuous flow
A continuous flow organocatalytic synthesis of formamides has been developed using amines and CO2 reaction partners in the presence of a silane reductant. The process is conducted under comparatively mild reaction conditions and allows the preparation of a broad family of N-substituted formamides in good yield. The heterogenized catalyst is a PS-supported superbase (DBU), while (MeO)3SiH proved to be the most efficient reductant. The reaction was first optimized in batch mode, giving access to fifteen different N-substituted formamide products. The optimized conditions were then used as input to develop a proof-of-concept continuous flow process based on the model substrate N-phenyl aniline thereby providing N-methyl-N-phenyl formamide as target with good productivity.
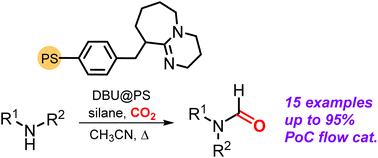
Zanda, N.; Primitivo, L.; Chaudhari, M.; Kleij, A. W.; Pericàs, M. À.
Org. Chem. Front. 2023, (10), 375-381
DOI:
10.1039/d2qo01711c

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















