Nanoconfinement of polyoxometalates in cyclodextrin: computational inspections of the binding affinity and experimental demonstrations of reactivity modulation
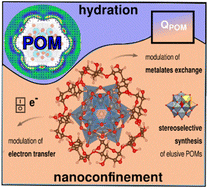
Chaotropic polyoxometalates (POMs) form robust host-guest complexes with gamma-cyclodextrin (gamma-CD), offering promising applications in catalysis, electrochemical energy storage, and nanotechnology. In this article, we provide the first computational insights on the supramolecular binding mechanisms using density-functional theory and classical molecular dynamics simulations. Focusing on the encapsulation of archetypal Keggin-type POMs (PW12O403-, SiW12O404- and BW12O405-), our findings reveal that the lowest-charged POM, namely PW12O403- spontaneously confines within the wider rim of gamma-CD, but BW12O405- does not exhibit this behaviour. This striking affinity for the hydrophobic pocket of gamma-CD originates from the structural characteristics of water molecules surrounding PW12O403-. Moreover, through validation using 31P NMR spectroscopy, we demonstrate that this nanoconfinement regulates drastically the POM reactivity, including its capability to undergo electron transfer and intermolecular metalate Mo/W exchanges. Finally, we exploit this nanoconfinement strategy to isolate the elusive mixed addenda POM PW11MoO403-. The water structure surrounding polyoxometalates dictates their capacity to form host-guest complexes with cyclodextrins.
Segado-Centellas, M.; Falaise, C.; Leclerc, N.; Priso, G. M.; Haouas, M.; Cadot, E.; Bo, C.
Chem. Sci. 2024, 15, 15849-15857
DOI:
10.1039/d4sc01949k

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements




















