Michael acceptor properties of 6-bicycloaryl substituted (R)-5,6-dihydro-2H-pyran-2-ones
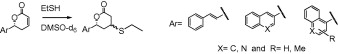
The mechanism of action for α,β-unsaturated lactones can be explained by their Michael acceptor properties. They have the potential of being covalently binding inhibitors by accepting nucleophiles from target proteins. In this work, Michael addition reactions of ethanethiol with 6-bicycloaryl substituted 5,6-dihydro-2H-pyran-2-ones were studied to explore the existence of such interactions. Three of the Michael addition products were isolated and tested over PC3 (human prostate cancer) and MCF-7 (human breast adenocarcinoma) cancer cell lines and no cytotoxicity was observed. It was revealed that biological activity depends on the existence of a Michael acceptor, but potency probably depends upon the 3D structure of the substituent on lactone ring. The primary chemical-quantum properties of the lactones were also calculated using the Spartan’08 computer program.
P. Kasaplar, Ö. Yılmazer and A.Çağır
Bioorg. Chem. 2010, 38, 186-189

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements


















