Mechanism-performance relationships of metal oxides in catalyzed HCl oxidation
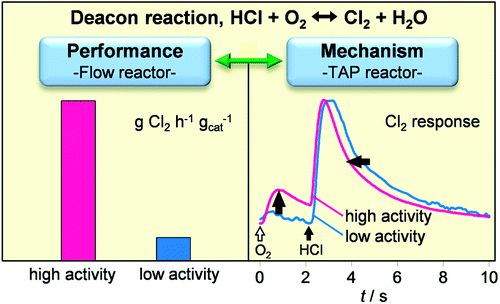
The gas-phase oxidation of HCl to Cl2 over heterogeneous catalysts, known as the Deacon process, is a sustainable way for chlorine recycling in the chemical industry. Mechanistic aspects of this reaction over metal oxides (Cr2O3, CeO2, and MnO2) have been gathered using the temporal analysis of products (TAP) reactor and compared with the outcome of previous studies over RuO2 and CuO. The intrinsic features of the TAP technique enable investigation of this demanding reaction in a safe manner and under highly controlled conditions. We have correlated the catalytic activity measured isothermally in a continuous-flow reactor at ambient pressure with mechanistic descriptors derived from the transient responses of reaction products (Cl2 and H2O) in the TAP reactor. The order of activity was RuO2 > Cr2O3 > CeO2 CuO > MnO2. The oxides with lowest activity, MnO2 and CuO, exhibited bulk chlorination detected by X-ray diffraction and a highly impeded Cl2 evolution. Chlorination of Cr2O3 and CeO2 during reaction conditions was limited to the surface, as observed with RuO2. However, catalyst reoxidation over the former two catalysts is more costly. Consequently, RuO2 possesses the two main features for a suitable Deacon catalyst: limited chlorination, conferring stability; and easier Cl2 evolution, allowing low-temperature operation.
A. P. Amrute, C. Mondelli, M. A. G. Hevia, J. Pérez-Ramírez
ACS Catal. 2011, 1, 583-590

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements


















