Mechanically Constrained Catalytic Mn(CO)3Br Single Sites in a Two-Dimensional Covalent Organic Framework for CO2 Electroreduction in H2O
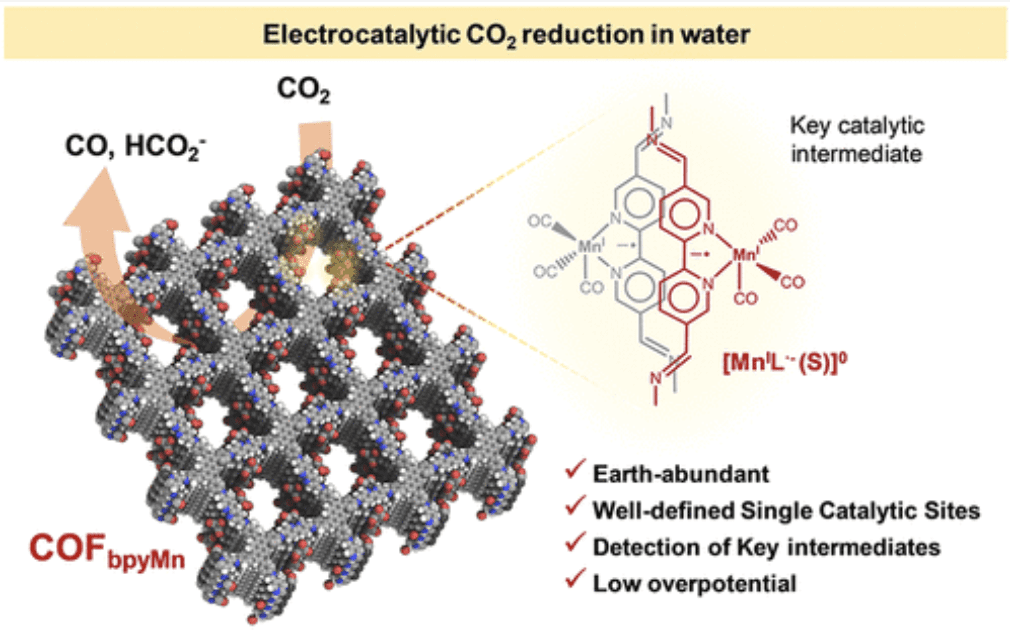
The development of CO2 electroreduction (CO2RR) catalysts based on covalent organic frameworks (COFs) is an emerging strategy to produce synthetic fuels. However, our understanding on catalytic mechanisms and structure–activity relationships for COFs is still limited but essential to the rational design of these catalysts. Herein, we report a newly devised CO2 reduction catalyst by loading single-atom centers, {fac-Mn(CO)3S}, (S = Br, CH3CN, H2O), within a bipyridyl-based COF (COFbpyMn). COFbpyMn shows a low CO2RR onset potential (η = 190 mV) and high current densities (>12 mA·cm–2, at 550 mV overpotential) in water. TOFCO and TONCO values are as high as 1100 h–1 and 5800 (after 16 h), respectively, which are more than 10-fold higher than those obtained for the equivalent manganese-based molecular catalyst. Furthermore, we accessed key catalytic intermediates within a COF matrix by combining experimental and computational (DFT) techniques. The COF imposes mechanical constraints on the {fac-Mn(CO)3S} centers, offering a strategy to avoid forming the detrimental dimeric Mn0–Mn0, which is a resting state typically observed for the homologous molecular complex. The absence of dimeric species correlates to the catalytic enhancement. These findings can guide the rational development of isolated single-atom sites and the improvement of the catalytic performance of reticular materials.
Dubed Bandomo, G. C.; Sekhar Mondal, S.; Franco, F.; Bucci, A.; Martin-Diaconescu, V.; Ortuño, M. A.; van Langevelde, P. H.; Shafir, A.; López, N.; Lloret-Fillol, J.
ACS Catal. 2021, 11, 7210–7222
DOI:
10.1021/acscatal.1c00314

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements





















