Enhanced binding of methyl alkylammonium cations through preorganization of a water-soluble calix[4]pyrrole
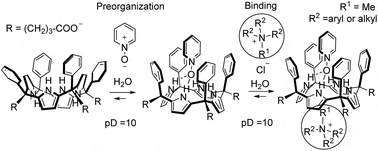
We describe the synthesis of two tetra-alpha aryl-extended calix[4]pyrroles (C[4]Ps) 4a-b bearing four terminal carboxylic groups in their meso-propyl chains defining the lower rims. The synthesized C[4]Ps became soluble (1-3 mM) in water at pD = 10. We probed the interaction of 4a towards tetra-methylammonium (G1) chloride in water using 1H NMR spectroscopy. The C[4]P 4a includes G1 in the shallow aromatic cavity defined by the pyrrole rings in cone conformation forming a 1 : 1 complex G1 subset of 4a. Pyridine-N-oxide (PNO) binding in the larger polar aromatic cavity of 4a results in the quantitative self-assembly of the supramolecular receptor PNO@4a featuring the pyrrole rings preorganized in cone conformation. The PNO@4a receptor displays improved binding properties towards G1 than the parent C[4]P 4a. We thermodynamically characterized (1H NMR titrations and ITC experiments) the 1 : 1 complexes of PNO@4a with a series of tetra-alkylammonium salts, including biologically relevant examples. The PNO@4a supramolecular receptor displays significant affinity (log K = 3-4) but lacks selectivity in water binding of methyl trialkyl ammonium cations. Cation-pi and coulombic interactions are the main intermolecular forces stabilizing the complexes. We also performed DFT calculations to gain some insights into the complexes’ structures. The binding of pyridine-N-oxide with a calix[4]pyrrole results in the self-assembly of a preorganized supramolecular receptor displaying significant affinity (log K = 3-4) for the binding of tetra-alkylammonium cations in water.
Valencia, E.; Ballester, P.
Org. Biomol. Chem. 2024,
DOI:
10.1039/d4ob00843j

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















