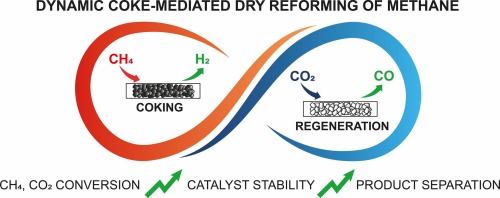
Enabling complete conversion of CH4 and CO2 in dynamic coke-mediated dry reforming (DC-DRM) on Ni catalysts
Dynamic coke-mediated dry reforming of methane (DC-DRM) is an unsteady-state strategy to overcome the limitations of co-feed operation, including the fast deactivation of the catalysts and the loss of valuable H2 in the reverse water gas-shift reaction. This paper proves the feasibility of DC-DRM on Ni-based catalytic systems, identifying suitable metal oxides supports and evaluating the role of metallic promoters. A La-promoted Ni/ZrO2 catalyst exhibited excellent and stable catalytic performances at 800 degrees C approaching complete conversion of the CH4 and CO2 reactant pulses in the reaction loop, and separation of the H2 and CO product streams. Adding the redox functionality of reducible oxides (TiO2) in the catalyst support is demonstrated as a powerful tool to enable direct formation of syngas in the methane pulse with control on the H2/CO ratio.
D. Pinto, L.J. Hu, A. Urakawa
Chem. Eng. J. 2023, 474, 145641
DOI:
10.1016/j.cej.2023.145641

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements


















