Dimensionality reduction of complex reaction networks in heterogeneous catalysis: From linear‐scaling relationships to statistical learning techniques
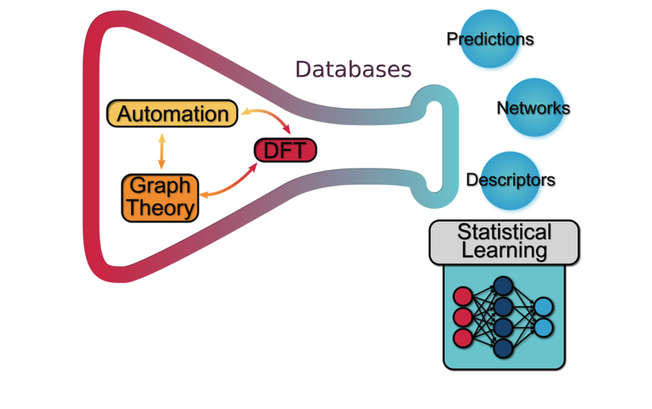
The mechanistic analysis in heterogeneous catalysis is based on listing all elementary steps and evaluating explicitly their energies. To this end, computational models based on Density Functional Theory have become a standard to estimate the information needed in mechanistic studies. Typically, either the minimum energy paths or those with the smaller span are summarized in reaction profiles. Such simplifications gather a lot of information, although further dimensionality reduction is required to obtain the most relevant descriptors of catalytic activity and generate the so‐called volcano plots. The selection of descriptors has been traditionally based on simple intermediates, such as central atoms in small molecules (as C in CH4), which have good thermodynamic correlations to other fragments containing them. Yet, in emerging processes (recent studies), the number of intermediates involved increase, configurational effects and lateral interactions become significant, and complex materials with low symmetry are employed, thus the simple rules encapsulated in linear scaling relationships lose their predictive power due to error accumulation. At the same time, large datasets generated for the intermediates call for statistical analysis and thus these techniques are being leveraged to chemical systems, particularly to reduce their dimensionality.
Pablo‐García, S.; García‐Muelas, R.; Sabadell‐Rendón, A.; López, N.
Wiley Interdiscip. Rev. Comput. Mol. Sci. 2021, 11 (6), e1540
DOI:
10.1002/wcms.1540

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















