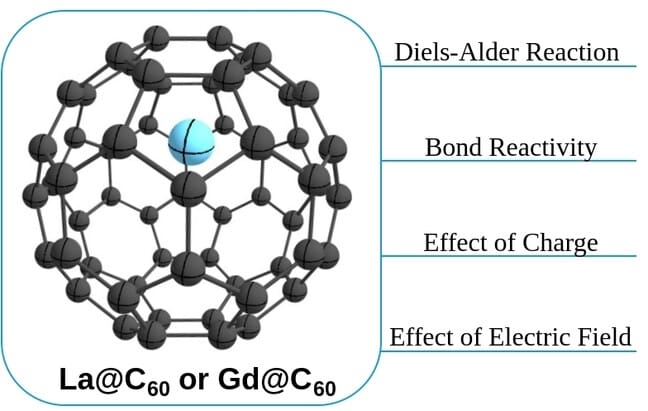
Diels-Alder Reaction Mechanisms of La@C60 and Gd@C60 Studied Using Density Functional Theory
Encapsulation of transition metals represents a crucial method for modifying the electronic structure and regulating the reactivity of fullerene, thereby expanding its applications. Herein, we present calculations with density functional theory methods to investigate the mechanisms of the Diels-Alder (DA) reactions of cyclopentadiene and La@C-60 or Gd@C-60 as well as their tricationic derivatives. Our findings indicate that the encapsulation of La and Gd into the C-60 cage is thermodynamically favorable. The DA reactions are favored by the presence of La and Gd, with lower barriers, though the regioselectivity, favoring 6-6 bonds in the fullerene, is not affected. The effect of external electric fields has been also considered.
Cui, C.-X.; He, J.-R.; Qu, L.-B.; Li, C.-X.; Peng, J.-L.; Maseras, F.
Chem.-Eur. J. 2024,
DOI:
10.1002/chem.202402572

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















