Development of a robust immobilized organocatalyst for the redox-neutral mitsunobu reaction
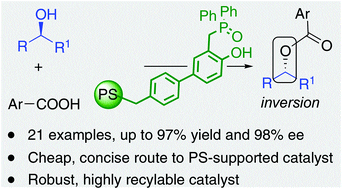
A polystyrene-supported version of the Denton catalyst for redox-neutral Mitsunobu reactions, (2-hydroxybenzyl)diphenylphosphine oxide, has been developed and used in catalytic inversion of enantiopure secondary alcohols (21 examples, up to 97% yield and 98% ee) with 2-nitrobenzoic and 2,4-dinitrobenzoic acids. The use in the reaction of alternative pronucleophiles has also been explored (8 successful and 3 unsuccessful examples). The functional resin shows high recyclability (10 cycles, 30 days operation) and can be re-activated by simple treatment with butylamine with further extension of its useful life.
Zhou, L.; Perulli, S.; Mastandrea, M. M.; Llanes, P.; Laia, J.; Pericàs, M. A.
Green Chem. 2021, 23, 8859-8864
DOI:
10.1039/D1GC02819G

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















