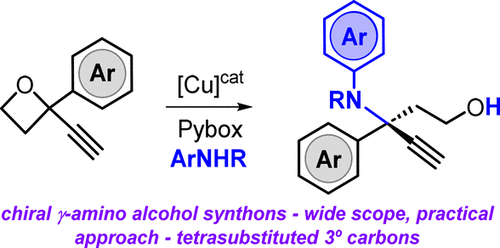
Cu-Catalyzed Asymmetric Synthesis of γ-Amino Alcohols Featuring Tertiary Carbon Stereocenters
Alkyne-functionalized oxetanes are presented as versatile substrates that in combination with amine reagents can be transformed into structurally diverse, chiral γ-amino alcohols featuring a tetrasubstituted tertiary stereocenter under Cu catalysis. Control experiments demonstrate the privileged nature of these oxetane precursors in terms of yield and asymmetric induction levels in the developed protocol, and postsynthetic modifications offer an easy way to access more advanced synthons.
Delgado, A.; Orlando, P.; Lanzi, M.; Benet-Buchholz, J.; Passarella, D.; Kleij, A. W.
Org. Lett. 2024, 26 (36), 7596-7600
DOI:
10.1021/acs.orglett.4c02682

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















