Comproportionation and disproportionation in nickel and copper complexes
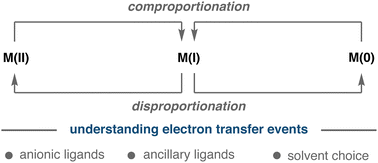
Disproportionation and comproportionation reactions have become increasingly important electron transfer events in organometallic chemistry and catalysis. The renewed interest in these reactions is in part attributed to the improved understanding of first-row metals and their ability to occupy odd and even oxidation states. Disproportionation and comproportionation reactions enable metal complexes to shuttle between various oxidation states, a matter of utmost relevance for controlling the speciation and catalytic turnover. In addition, these reactions have a direct impact in the thermodynamic and kinetic stability of the corresponding metal complexes. This review covers the relevance and impact of these processes in electron transfer reactions and provides valuable information about their non-negligible influence in Ni- and Cu-catalysed transformations. This review covers factors that contribute to comproportionation and disproportionation reactions in transition metal complexes and provide insight into the importance of these electron transfer events in Ni- and Cu-catalyzed transformations.
C. S. Day, R. Martin
Chem. Soc. Rev. 2023, 52 (19), 6601-6616
DOI:
10.1039/d2cs00494

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















