Accelerated study of the citral-acetone condensation kinetics over activated Mg-Al hydrotalcite
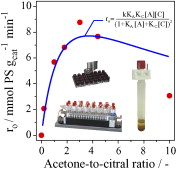
The kinetics of the citral-acetone condensation to pseudoionone (PS) over activated Mg-Al hydrotalcite (meixnerite) was studied in a 16 parallel-batch reactor set-up varying the temperature, catalyst amount, and molar acetone-to-citral (A/C) ratio. Tests with different stirring speeds and particle sizes were conducted in order to exclude the presence of mass transport limitations. The A/C ratio is a key reaction descriptor. The initial rate of pseudoionone formation exhibits a maximum at A/C = 3, leading to a PS selectivity of 100%. At higher or lower A/C ratios, diacetone alcohol is the main by-product and at A/C < 2 the product of citral self-condensation is additionally formed. Due to this, the pseudoionone selectivity at extreme A/C ratios decreases to 70-80%. The amount of carbonaceous deposits was determined by thermogravimetry and increased as the A/C ratio decreased. A Langmuir-Hinshelwood model involving acidic Al3+ and basic OH- sites and the surface reaction as rate-determining step described well the experimental data. The position of the optimal A/C ratio is determined by the relative ratio of adsorption constants of the reactants on the catalyst, being one order of magnitude larger in citral than in acetone. This feature can be generalized to aldol reactions involving other substrates.
S. Abelló, S. Dhir, G. Colet, J. Pérez-Ramírez
Appl. Catal. A-Gen. 2007, 325, 121-129

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements


















