A visible-light mediated three-component radical process using dithiocarbamate anion catalysis
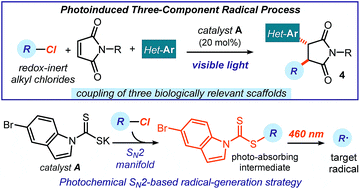
We report a photoinduced three-component radical process, which couples readily available alkyl chlorides, maleimides, and heteroaromatic fragments to rapidly generate complex chiral products with high diastereocontrol. This method generates radicals via an SN2-based photochemical catalytic mechanism, which is not reliant on the redox properties of the precursors. It therefore grants access to open-shell intermediates from substrates that would be incompatible with or inert to classical radical-generating strategies. The redox-neutral conditions of this process make it tolerant of redox-sensitive substrates and allow the installation of multiple biologically relevant heterocycles within the cascade products.
Cuadros, S.; Horwitz, M. A.; Schweitzer-Chaput, B.; Melchiorre, P.
Chem. Sci. 2019, 10, 5484–5488
DOI:
10.1039/C9SC00833K

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















