Water Oxidation Electrocatalysis in Acidic Media with Fe-Containing POMs/Carbon Composites
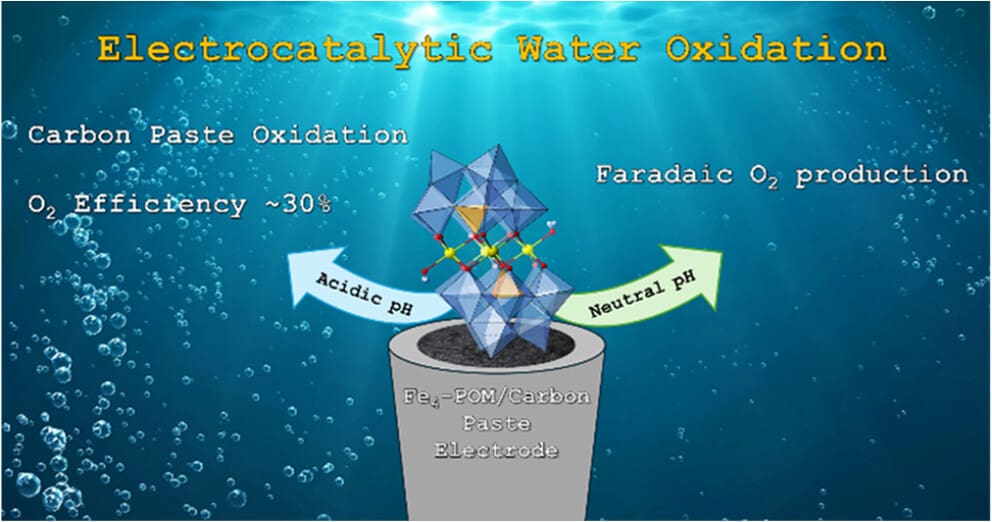
Iron-based catalysts are very appealing in terms of applications due to the low cost of Fe and to its abundance in the Earth’s crust. In the field of water oxidation, unfortunately, iron oxides cannot match the activity of Co or Ni oxides, much less than the activity of noble metal oxides (IrO2). The activity of transition metals to promote the oxygen evolution reaction (OER) can be tuned and enhanced by their incorporation into polyoxometalate frameworks (POMs). In comparison with metal oxides, POMs offer a controlled, discrete structure and a tailor-made environment. Fe-POMs still show a low OER activity in neutral or basic media when compared to Co-POMs. When moving to highly acidic media, we have found an unexpected electrochemical response in carbon paste electrodes containing salts of the [Fe4III(H2O)2(PW9O34)2]6– (Fe4) polyanion. In oxidative conditions, these electrodes showed lower onset potentials and higher current densities than their Co-based analogues, contrary to computational expectations. Careful analyses have shown the excellent stability of the Fe4 in these pH < 1 conditions, but a poor selectivity. CO2 is the dominant product, in addition to O2. The capability of Fe4 to oxidize amorphous carbon under acidic conditions appears to be unique since it is not found in Fe oxides or simple Fe salts. Thus, Fe-POMs, in acidic conditions, are still modest OER catalysts, but exhibit a unique performance when electrochemically oxidizing carbon.
Azmani, K.; Besora, M.; Yu, J.; Teillout, A.-L.; de Oliveira, P.; Mbomekallé, I.-M.; Soriano-López, J.; Poblet, J. M.; Galán-Mascarós, J. R.
Inorg. Chem. 2025, 64 (9), 4260–4266
DOI:
10.1021/acs.inorgchem.4c04422
Associated projects:
-
NEWBOUND
Electrolysis is a promising technology to support the industrial decarbonization. Substitution of fossil by green fuels obtained from renewable energy and electrolyzers could transform the energy cycle, allowing for a rapid transition towards sustainable processes. Electrolysis advantages go even further. It could also contribute to the industrial electrosynthesis of chemical commodities. However, improved low-cost, fast and efficient processes are needed. And also highly selective to attempt the large scale electrosynthesis of chemical products. Our research team has studied electrocatalysts for the oxygen evolution reaction (OER), the bottleneck for the production of green hydrogen from water.
See more

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements





















