Water-hydroxide trapping in cobalt tungstate for proton exchange membrane water electrolysis
The oxygen evolution reaction is the bottleneck to energy-efficient water-based electrolysis for the production of hydrogen and other solar fuels. In proton exchange membrane water electrolysis (PEMWE), precious metals have generally been necessary for the stable catalysis of this reaction. In this work, we report that delamination of cobalt tungstate enables high activity and durability through the stabilization of oxide and water-hydroxide networks of the lattice defects in acid. The resulting catalysts achieve lower overpotentials, a current density of 1.8 amperes per square centimeter at 2 volts, and stable operation up to 1 ampere per square centimeter in a PEMWE system at industrial conditions (80°C) at 1.77 volts; a threefold improvement in activity; and stable operation at 1 ampere per square centimeter over the course of 600 hours.
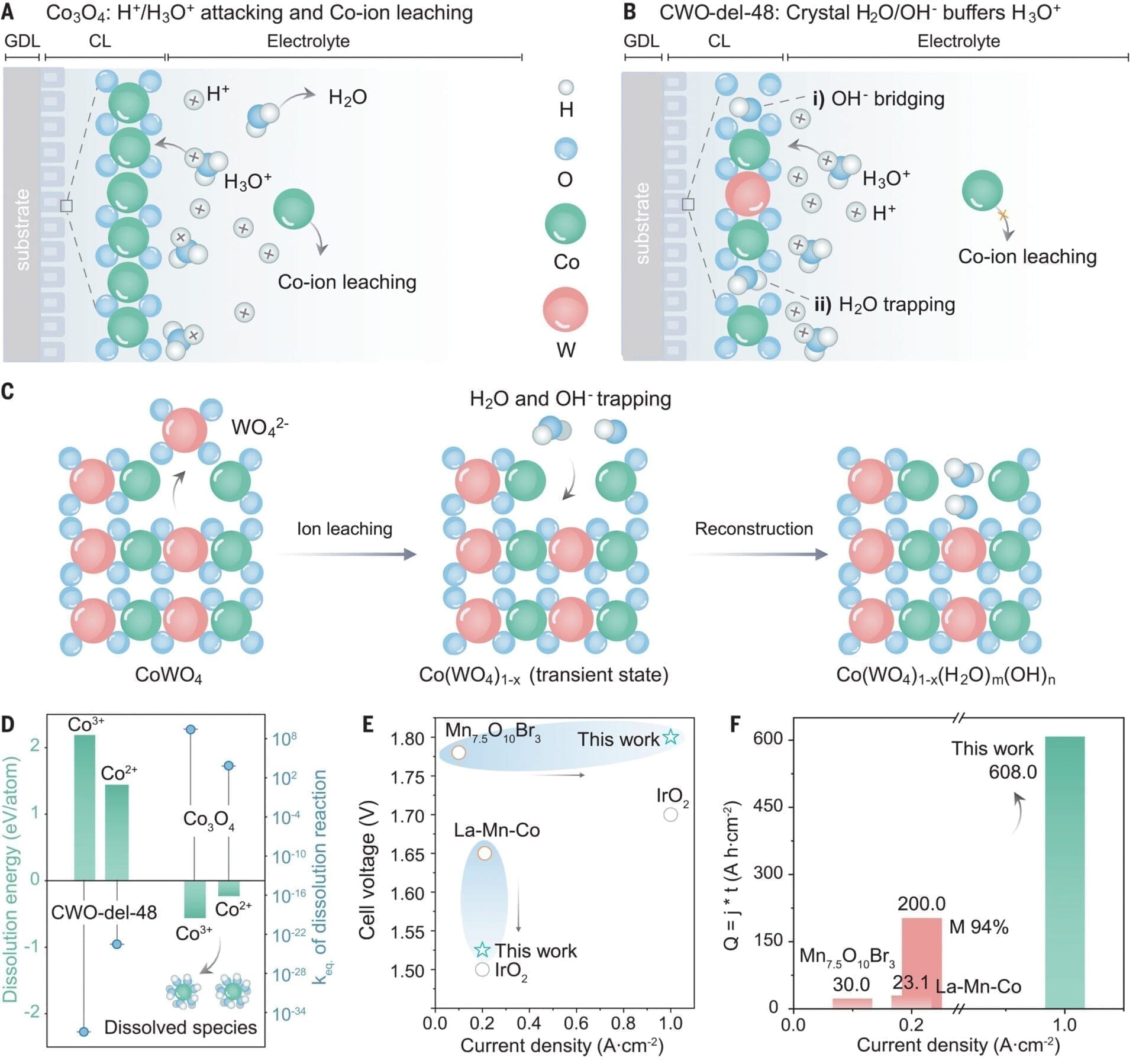
Ram, R.; Xia, L.; Benzidi, H.; Guha, A.; Golovanova, V.; Garzón Manjón, A.; Llorens Rauret, D.; Sanz Berman, P.; Dimitropoulos, M.; Mundet, B.; Pastor, E.; Celorrio, V.; Mesa, C. A.; Das, A. M.; Pinilla-Sánchez, A.; Giménez, S.; Arbiol, J.; López, N.; García de Arquer, F. P.
Science 2024, 384 (6702), 1373-1380
DOI:
10.1126/science.adk984

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements




















