Synthesis of Daucane Natural Products Enabled by a Gold(I)-Catalyzed Tandem Cycloisomerization/(4 + 3) Cycloaddition
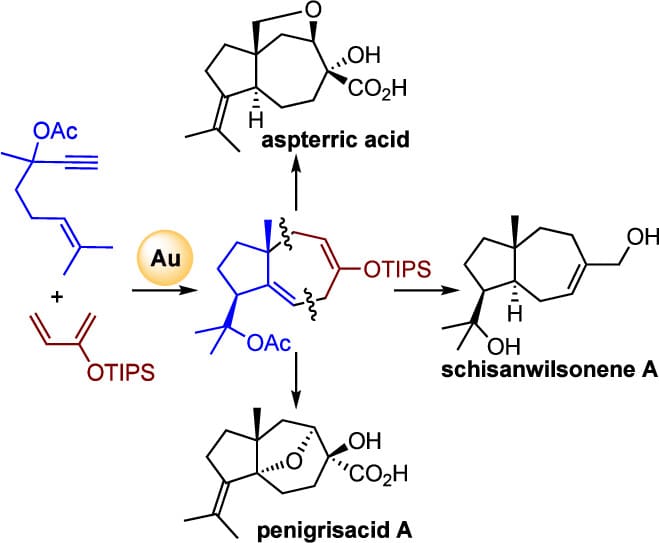
A divergent synthesis of three members of the daucane family of natural products is reported, enabled by a gold(I)-catalyzed cycloisomerization/formal (4 + 3) cycloaddition as the key step. The synthesis of penigrisacid A features a vanadium-catalyzed tandem epoxidation/SN2′ cyclization, whereas a Suárez radical cyclization enables the synthesis of aspterric acid. This work has also led to the reassignment of the structure of penigrisacid A as well as a short formal synthesis of schisanwilsonene A.
Martí, À.; Armengol-Relats, H.; Sadurní, A.; Pérez-Puigdomènech, M. À.; Echavarren, A. M.
Org. Lett. 2025
DOI:
10.1021/acs.orglett.4c04542

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements




















