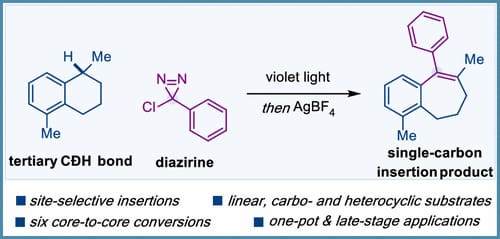
Single-Carbon Insertion into Single C-C Bonds with Diazirines
A novel platform for the skeletal editing of single C-C bonds via a single-carbon insertion has been developed using diazirines. This strategy involves the photogeneration of arylchlorocarbenes as carbynoid species that undergo site-selective carbene insertion into tertiary C-H bonds and a subsequent Wagner-Meerwein rearrangement promoted by a silver salt. Our skeletal editing strategy based on a formal selective carbyne C-C bond insertion has been demonstrated in six core-to-core conversions, including linear and cyclic benzylic substrates, alkanes and late-stage functionalizations.
Alfonso, V. G.; de la Vega-Hernández, K.; Suero, M. G.;
J. Am. Chem. Soc. 2024
DOI:
10.1021/jacs.4c12632
Associated projects:
-
CARBYNE
The art of organic synthesis and reaction discovery relies on logic-guided thought processes that often involve hypovalent carbon reactive species and their corresponding stabilised equivalent forms. However, not all of the possible carbon reactive intermediates and their reactivity rules have received the same attention by the synthetic community. Carbyne is a case in point. This is mainly because of the perceived lack of synthetic utility and challenges associated with controlling its extreme reactivity and lack of efficient sources. The EU-funded CARBYNE project is developing novel catalytic pathways for the production of carbyne equivalents and related species, to enhance applications of these unique and promising carbon reactive species in numerous fields.
See more -
FUN-C
The skeletal editing of molecules through the chemo- regio- and enantioselective manipulation of their CC bonds represents a longstanding challenge in synthetic organic chemistry. Clearly, the field of CC bond functionalization lags behind the more advanced area that uses relatively inert CH bonds for the construction of chiral enantioenriched molecules.
See more

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements






















