Practical Synthesis of Chiral Ferrocenenylphosphino-Gold(I) Catalysts and NEST Analysis of the Enantioinduction
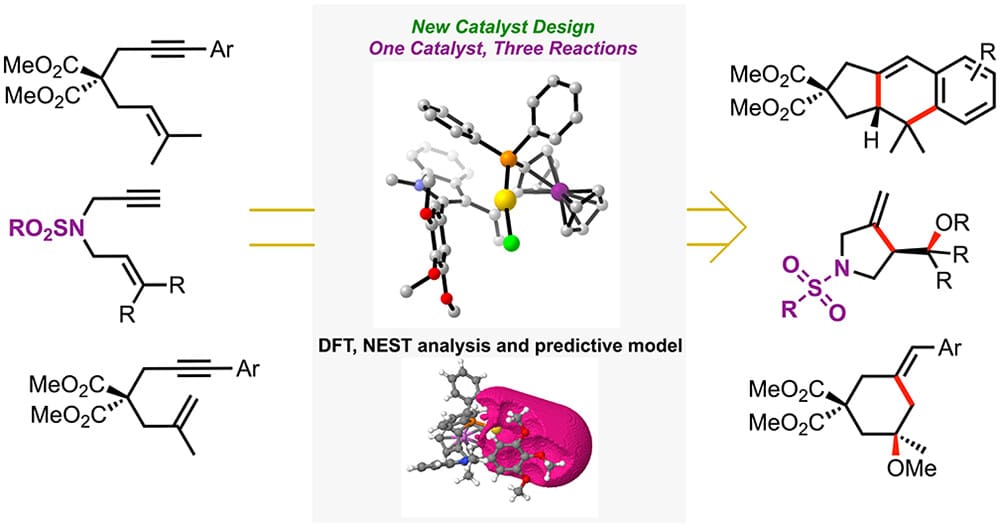
The concise modular synthesis of a family of monodentate 1,2-disubstituted ferrocene ligands containing a diaryl phosphine and a 2-aryl indole is described. Their gold(I) complexes were applied to the enantioselective gold(I)-catalyzed formal [4 + 2] cycloaddition of 1,6-arylenynes, the enyne cyclization/nucleophile addition of N-tethered 1,6-enynes, and the methoxycyclization of 1,6-arylenynes with high levels of enantioselectivity in all cases. Crystallographic and computational studies highlighted the relevant role of noncovalent interactions within the ligand scaffold and between the ligand and substrate in the modes of enantioinduction in the cyclization of unsaturated substrates. Our recently developed open-source tool NEST was applied to analyze the chiral pockets of the catalysts, which in combination with RDKit allowed us to understand the enantioselectivity in these reactions, paving the way for a predictive-based approach toward the rational development of chiral ligands for enantioselective Au(I) catalysis.
Mora, P.; Escofet, I.; Besora, M.; Cester Bonati, F.; Echavarren, A. M.
ACS Catal. 2025, 15, 2342–2350
DOI:
10.1021/acscatal.4c07495
Associated projects:
-
INTCATGOLD
With this project, we will contribute to the advancement of the field of gold homogeneous catalysis by synthesizing advanced chiral gold catalysts that mimic the characteristics of enzymes and to develop new synthetic methods based on the activation of acetylene gas, a commodity in industry.
See more -
Foldmetcat
The goal of this proposal is to design new types of catalysts containing electrophilic transition metal centers that could simultaneously fold and activate polyunsaturated substrates promoting non-inherent cyclization modes. These unconventional cyclisation cascades challenge existing research that views the substrate intrinsic reactivity as the sole factor determining chemical reactivity of carbocation-initiated processes. They also provide access to large carbocyclic skeletons such as those present in the enantioselective synthesis of taxol and ophiobolin. Another aim is to develop general-purpose, efficient chiral electrophilic catalysts based on metals different from gold.
See more -
Organometallic Chemistry in Organic Synthesis
We work on the development of organic chemistry, exploring new areas of organometallic chemistry. After our early work on palladium, nickel, ruthenium, and platinum, our most recent contributions have been in homogeneous gold catalysis, where we established the foundations that have guided the discovery of new transformations by other research groups.
See more

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements