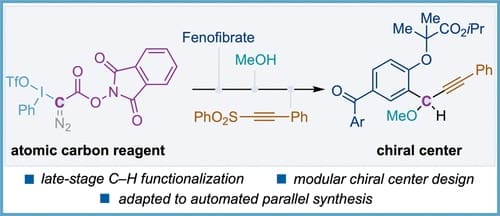
Late-Stage Photoredox-Catalyzed Aryl C–H Bond Diazomethylation with Atomic Carbon Reagents
Herein, we present a novel class of diazo compounds as atomic carbon reagents substituted with two orthogonal redox-active leaving groups that were exploited in the late-stage construction of chiral centers with aryl C–H bonds from aromatic feedstocks and drug molecules. Key to the strategy was the use of photoredox catalysis to enable an initial C–H diazomethylation reaction able to generate diazomethyl-substituted redox-active esters. Subsequent construction of chiral centers with readily available starting materials proceeded using a broad range of well-known diazo and redox-active ester functionalizations. Moreover, the applicability of our novel atomic carbon reagent was tested in the automated parallel synthesis of a library of Fenofibrate derivatives.
Puggioli, A.; Jiang, L.; Herraiz, A. G.; Nannini, L. J.; de la Vega-Hernández, K.; Rey-Blanco, A.; Diéguez-Vázquez, A.; Cañellas, S.; Suero, M. G.
J. Am. Chem. Soc. 2025
DOI:
10.1021/jacs.5c00045

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements




















