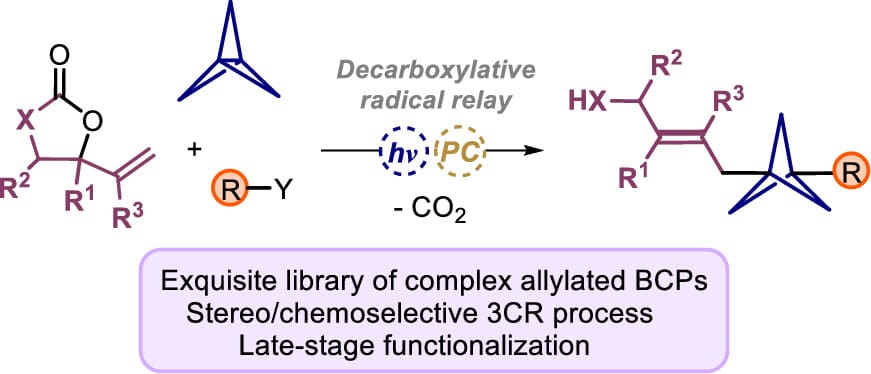
Highly Functional Allyl-Bicyclo[1.1.1]pentane Synthesis by Radical-Initiated Three-Component Stereoselective Allylation
Rapid access to highly functional allylated BCP synthons can be achieved with good selectivity and yield through a radical, three-component reaction (3CR) regime using various combinations of radical precursors and vinyl-appended heterocycles acting as versatile and modular precursors. This practical process combines mild operating conditions, a wide scope of reaction partners, and the ability to diversify the functionalized allylic scaffolds further using the allyl and other functional groups as synthetic branching points. The developed protocol allows structural alteration and increases the molecular complexity through late-stage drug modifications and drug conjugation approaches. Mechanistic probes demonstrate that the 3CR process is initiated by a selective, light-promoted radical addition to [1.1.1]-propellane, followed by coupling with the vinyl-substituted heterocycle, which represents a formal decarboxylative radical addition/double bond relay/protonation sequence.
Zeng, Q.; Shi, W.; Kleij, A. W.
JACS Au 2025
DOI:
10.1021/jacsau.4c01129
Associated projects:
-
CONQUEST
This project, CONQUEST, will focus on new transition metal catalyzed approaches for the construction of quaternary and tetrasubstituted tertiary carbon stereocenters enabled through decarboxylative allylation reactions. Compounds featuring acyclic quaternary stereocenters are attractive targets as the development of new scaffolds with such a structural element are valuable to increase the chemical space that is exploited by medicinal and fine-chemical chemists to develop new and/or improved drug molecules.
See more -
AVANT-GARDE
The AVANT-GARDE project aims to uncover new reactivity patterns and opportunities to transform vinyl-, alkynyl- and allene-appended heterocyclic substrates into stereodefined synthons with value in fine-chemical and pharmaceutical development programs. The key approximation is through the utilization of transition metal (TM) catalysis, and more specifically the use of (mostly) base metal (M = Ni, Co, Cu) and/or photocatalysts to assist these protocols. The project builds on the previous and extensive experience of the Kleij group in the formation of sterically challenging stereocenters empowered by TM catalysis.
See more

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements























