Catalytic transformation of carbon dioxide into seven-membered heterocycles and their domino transformation into bicyclic oxazolidinones
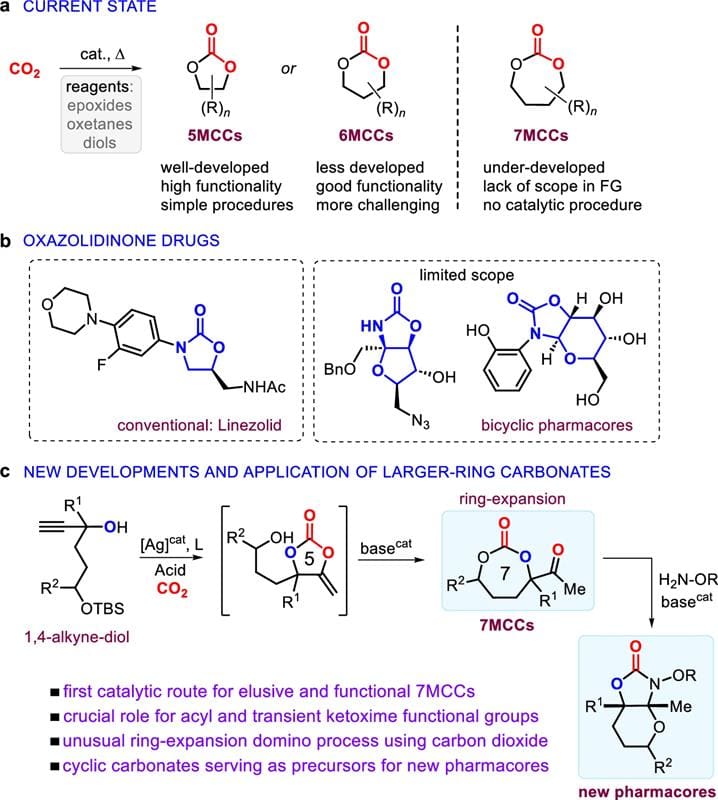
Converting carbon dioxide (CO2) into valuable heterocycles is of great synthetic value but is usually limited to five- and six-membered ring compounds. Here, we report a catalytic approach for transforming this carbon renewable into seven-membered heterocycles using a double-stage approach, combining a silver-catalyzed alkyne/CO2 coupling and a subsequent base-catalyzed ring-expansion. This methodology avoids the formation of thermodynamically more stable, smaller-ring by-products and has good functional group tolerance. The synthetic application of these larger-ring cyclic carbonates is further demonstrated by showing their unique ability to serve as synthons for the preparation of bicyclic oxazolidinone pharmacores through an intramolecular domino sequence that involves a transient ketimine group, and various other intermolecular transformations. The results described herein significantly expand on the use of CO2 as a cheap and versatile carbon feedstock generating elusive heterocycles and pharmaceutically relevant compounds.
Shi, W.; Benet-Buchholz, J.; Kleij, A. W.
Nat. Commun. 2025, 16, 1372
DOI:
10.1038/s41467-025-56681-5
Associated projects:
-
CONQUEST
This project, CONQUEST, will focus on new transition metal catalyzed approaches for the construction of quaternary and tetrasubstituted tertiary carbon stereocenters enabled through decarboxylative allylation reactions. Compounds featuring acyclic quaternary stereocenters are attractive targets as the development of new scaffolds with such a structural element are valuable to increase the chemical space that is exploited by medicinal and fine-chemical chemists to develop new and/or improved drug molecules.
See more -
AVANT-GARDE
The AVANT-GARDE project aims to uncover new reactivity patterns and opportunities to transform vinyl-, alkynyl- and allene-appended heterocyclic substrates into stereodefined synthons with value in fine-chemical and pharmaceutical development programs. The key approximation is through the utilization of transition metal (TM) catalysis, and more specifically the use of (mostly) base metal (M = Ni, Co, Cu) and/or photocatalysts to assist these protocols. The project builds on the previous and extensive experience of the Kleij group in the formation of sterically challenging stereocenters empowered by TM catalysis.
See more

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements























