Access to Highly Functional and Polymerizable Carbonate-Drug Conjugates
We here report the synthesis of two types of six-membered cyclic carbonate monomers equipped with various drug molecules through ester linkages. The target compounds can be isolated in good yields and feature diagnostic IR and 13C NMR spectroscopic fingerprints in line with their proposed connectivities. As a potential application, we investigated their ring-opening polymerization (ROP), showing that the nature of the cyclic carbonate is crucial towards macromolecular carbonate formation. The functionalized polycarbonates have molecular weights of up to 10 kg/mol, controllable functionality and a variable drug-to-carbonate ratio. This work demonstrates the adaptive synthesis of new types of functionalized six-membered cyclic carbonates with potential as precursors to polycarbonate-drug type macromolecules.
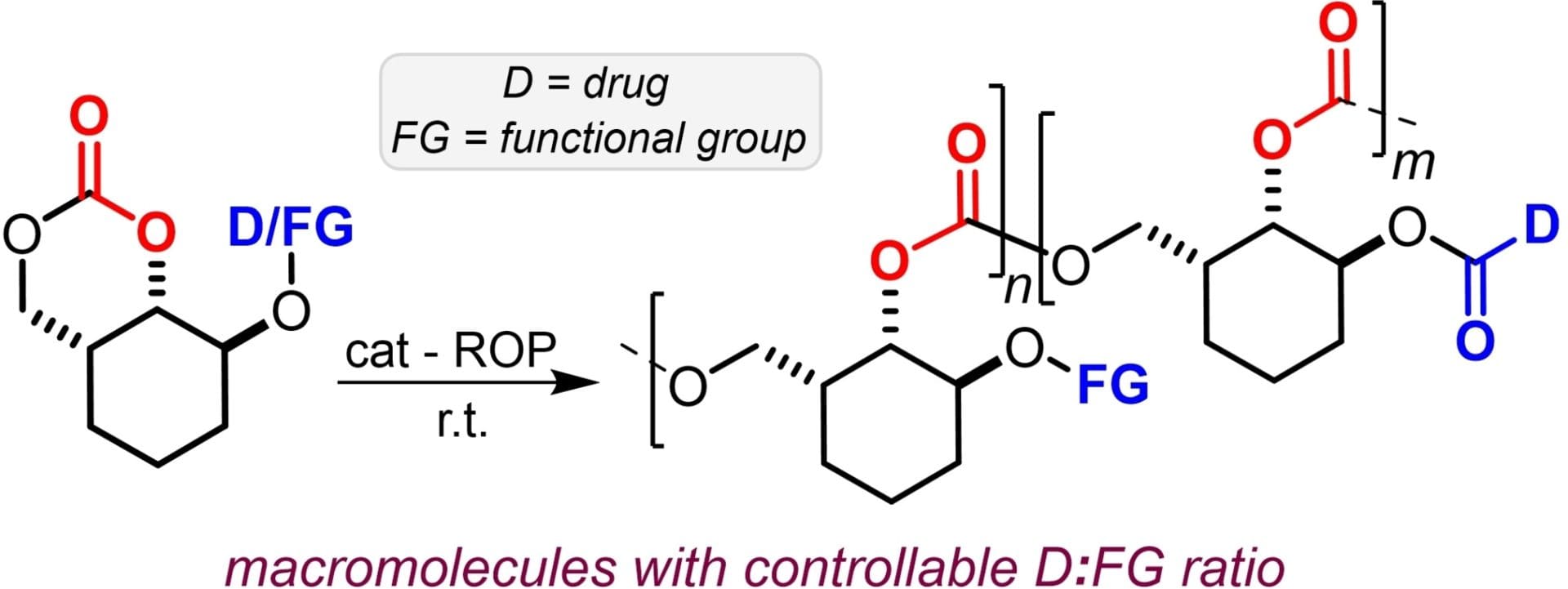
Shi, W.; Senthamarai, T.; Lanzi, M.; Orlando, P.; Nogués Martín, R.; Kleij, A. W.
ChemSusChem 2025, e202500031
DOI:
10.1002/cssc.202500031
Associated projects:
-
CONQUEST
This project, CONQUEST, will focus on new transition metal catalyzed approaches for the construction of quaternary and tetrasubstituted tertiary carbon stereocenters enabled through decarboxylative allylation reactions. Compounds featuring acyclic quaternary stereocenters are attractive targets as the development of new scaffolds with such a structural element are valuable to increase the chemical space that is exploited by medicinal and fine-chemical chemists to develop new and/or improved drug molecules.
See more -
AVANT-GARDE
The AVANT-GARDE project aims to uncover new reactivity patterns and opportunities to transform vinyl-, alkynyl- and allene-appended heterocyclic substrates into stereodefined synthons with value in fine-chemical and pharmaceutical development programs. The key approximation is through the utilization of transition metal (TM) catalysis, and more specifically the use of (mostly) base metal (M = Ni, Co, Cu) and/or photocatalysts to assist these protocols. The project builds on the previous and extensive experience of the Kleij group in the formation of sterically challenging stereocenters empowered by TM catalysis.
See more -
I2-ICIQ Impulsion
I2: ICIQ Impulsion will impulse the professional career of 12 highly talented international fellows through the development of an innovative research programme based on interdisciplinarity, internationality and intersectoriality. This research programme will include a mandatory secondment (from two to six months) in a different research entity (academic and/or non-academic).
See more -
Research Group Kleij
The Kleij research group focuses on the use of sustainable catalysis approaches to transform bio-based carbon feedstock into valuable fine chemicals and polymers. Our trajectory is based on in-house developed catalysts comprising of abundant, cheap and green metals (Fe, Al, and to some extent Co) or simple and accessible organic frameworks (N-heterocycles, polyols).
See more

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements























