A Unified Electro- and Photocatalytic CO2 to CO Reduction Mechanism with Aminopyridine Cobalt Complexes
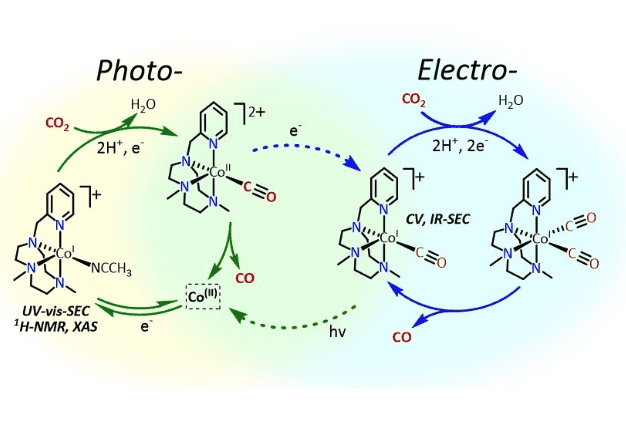
Mechanistic understanding of electro- and photocatalytic CO2 reduction is crucial to develop strategies to overcome catalytic bottlenecks. In this regard, herein it is presented a new CO2-to-CO reduction cobalt aminopyridine catalyst, a detailed experimental and theoretical mechanistic study toward the identification of bottle-necks and potential strategies to alleviate them. The combination of electrochemical and in-situ spectroelectrochemical (FTIR/UV-Vis SEC) studies together with spectroscopic techniques (NMR, EXAFS) lead us to identify elusive key electrocatalytic intermediates derived from complex [Co(pyMetacn)(OTf)2] (1) (pyMetacn = 1-[2-pyridylmethyl]-4,7-dimethyl-1,4,7-triazacyclononane) such as a highly reactive cobalt (I) (1(I)) and cobalt (I) carbonyl (1(I)-CO) species. 1(I) was obtained by electrochemical reduction of 1(II), and characterized by NMR, EXAFS and FTIR/UV-Vis SEC. The combination of spectroelectrochemical studies under CO2, 13CO2 and CO with DFT disclosed that 1(I) directly reacts with CO2 to form the pivotal 1(I)-CO intermediate at the 1(II/I) redox potential. At this redox potential the theoretical energy barrier for the C-O bond cleavage was found to be as low as 12.2 kcal·mol-1. However, the catalytic process does not proceed at the 1(II/I) redox potential, due to the formation of 1(I)-CO, which is a thermodynamic sink and the CO release restricts the electrocatalysis. In agreement with the experimental observed CO2-to-CO electrocatalysis at the 1(I/0) redox potential, computational studies suggested that the productive electrocatalytic cycle involves striking metal carbonyl intermediates such as [LN4Co0CO] (LN4 = pyMetacn), [LN4CoII(CO2)CO] and [LN4CoICO)2]. In contrast, under photochemical conditions, the catalytic process smoothly proceeds at the 1(II/I) redox potential. Under the latter conditions, it is proposed that the electron transfer rate is under diffusion control and then the CO release from 1(II)-CO is kinetically favored, facilitating the catalysis. Finally, we have found that visible light irradiation has a positive impact under electrocatalytic conditions. We envision that light irradiation can serve as an effective strategy to improve the CO2 reduction of molecular catalysts, via alleviating bottlenecks, such as the CO poisoning.
Fernández, S.; Franco, F.; Casadevall, C.; Martin-Diaconescu, V.; Luis, J.M.; Lloret-Fillol, J.
J. Am. Chem. Soc. 2020, 142 (1), 120-133
DOI:
10.1021/jacs.9b06633

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements



















