Stop mosquitoes!
Objective: To calculate the concentration of ammonia present in an insect bite product

-
Laboratory materials
Erlenmeyer
Burette
Beaker
-
Reagents
AFTER BITE®, 5 mL
Chlorhydric acid 0.5 M (HCl)
Methyl orange
Water
-
Safety
Don't forget the gloves, lab coat, and safety goggles!!!
-
Questions
Which reaction is taking place during the titration?
Calculate the mass concentration of ammonia present in the AFTER BITE® in mg/mL.
Procedure
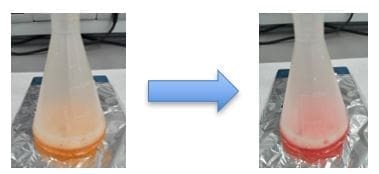
- In an Erlenmeyer flask, we add 5 mL of AFTER BITE® liquid, 25 mL of distilled water, and a few drops of methyl orange until it turns orange.
- We titrate this solution with constant stirring using 0.5 M hydrochloric acid until it permanently changes to a red color.
- We record the final volume of hydrochloric acid used.
Theoretical explanation
Ammonia is one of the most relevant chemical products. One of its numerous applications is to relieve the itching produced by mosquito bites.
The reaction taking place during the titration is:
NH3 + HCl NH4Cl
Knowing the volume of AFTER BITE® measured (2 mL), the concentration of thiosulfate (0.5 M), and the volume of thiosulfate needed to reduce all the iodine (burette reading), the concentration of ammonia in AFTER BITE® using can be calculated this formula:

With the concentration in mol/L, it is possible to convert it to mass concentration (g/L or mg/mL) considering that the molecular weight of ammonia is 17 g/mol.















