Playing CSI
Objective: Perform a luminescent reaction

-
Laboratory materials
2 Erlenmeyer flasks of 125 mL
A beaker of 250 mL
Spatulas
Graduated cylinders
-
Reagents
Luminol
Sodium hydroxide (NaOH)
Bleach (aprox. 5% NaOCl), 10 mL
Water
-
Safety
Don't forget the gloves, lab coat, and safety goggles!!!
-
Questions
What happens when the solution come into contact?
Which reaction takes place? Which type of reaction is it?
Procedure
- Mix 10 mL of bleach with 90 mL of water in a 125 mL Erlenmeyer flask.
- In the other Erlenmeyer flask, add a spatula tip of luminol, 1 lentil of NaOH, and 100 mL of water.
- Shake for one minute.
- Turn off the laboratory lights and simultaneously pour the two solutions into the beaker. Observe what happens
Theoretical explanation
In this experiment, a redox reaction takes place: a chemical reaction in which there is a transfer of electrons between the reacting species.
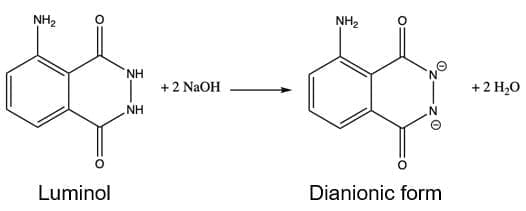
⦁ Hydroxide ions take 2 H+ from Luminol:

⦁ Then, the dianionic form of luminol gets oxidized by bleach NaClO and upon nitrogen liberations gives raise to the aminophthalate ion.
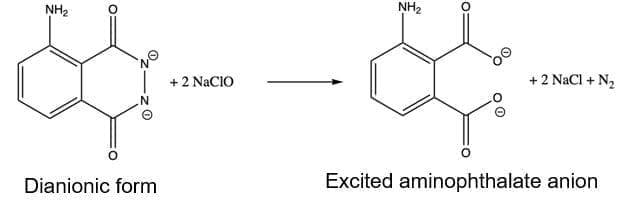
The aminophthalate ion formed is in its excited state. When it returns to its lower energy state, it releases this ‘excess’ of energy in the form of electromagnetic radiation (in this case, visible light).
NOTE: Solutions can be stored in the dark for several months.















