Chemical traffic light
Objective: Changing the color of a solution just by shaking it

-
Laboratory materials
1 Erlenmeyer
3 Spatulas
1 glass bottle with a cup
-
Reagents
Sodium hydroxide (NaOH)
Glucose
Indigo Carmine
Water
-
Safety
Don't forget the gloves, lab coat, and safety goggles!!!
-
Questions
What kind of reaction is taking place?
Where do all the colors come from?
Procedure
- Inside an Erlenmeyer flask, add 100 mL of distilled water.
- Weigh and add 1.5 g of NaOH and stir until it dissolves.
- Weigh and add 2 g of glucose and stir until they dissolve.
- Add a small amount of carmine indigo and mix well.
- Wait until the solution turns yellow, stir it, and observe what happens. The process may take a few minutes.
Theoretical explanation
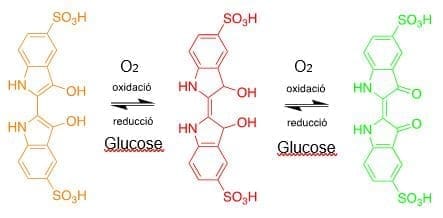
The colors observed in this experiment always come from indigo carmine. When the mixture is shaken, oxygen from the air enters and oxidizes indigo carmine first to its red form, and if we continue shaking, we can see the green form. When it stops stirring, the oxygen is consumed, and the indigo carmine reacts with the glucose present reducing it to the red form, and finally back to yellow. Sodium hydroxide is used as a base to facilitate all these redox reactions.
Here is a scheme of the redox reactions with the different colors of carmine indigo in basic medium: