Most read article in JACS and highlight in C&EN
Following the discovery of the novel and highly sought after reaction of cupration of fluoroform (that was another JACS’ most read paper), Vladimir Grushin‘s research group continues to report new applications of this fluoroform-derived CuCF3 reagent.
A month ago, ChemistryViews highlighted the group’s Angew. Chem. Int. Ed. report ‘Low Cost, Simple, Efficient and Safe Triflouromethylation of Aryl Boronic Acids’. Now, it is Chemical & Engineering News (C&EN) that covers in its October first issue (p. 52) the trifluoromethylation of α-haloketones communication that recently appeared in JACS.
The most read paper in JACS this month
As of now, this paper by Novák, Lishchynskyi and Grushin is the most read paper in JACS this month.
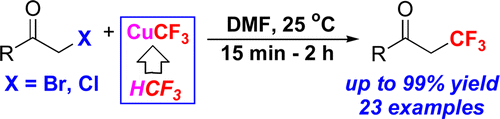
The communication describes the first nucleophilic α-trifluoromethylation reaction of carbonyl compounds, with fluoroform derived CuCF3, using only low-cost chemicals. The reaction proceeds at room temperature, exhibiting unprecedented functional group tolerance and furnishing the desired trifluoromethylated ketones in up to a 99% yield.
This new method is expected to find applications in the synthesis of biologically active compounds and specialty materials.
Related news

Let's create a brighter future
Join our team to work with renowned researchers, tackle groundbreaking
projects and contribute to meaningful scientific advancements






 27-05-2022
27-05-2022 


















